Rising Investment in Healthcare R&D
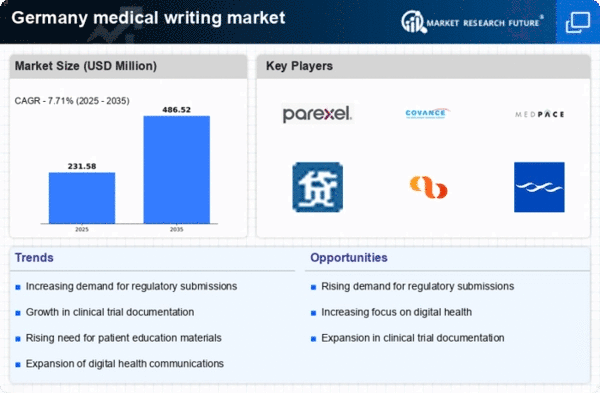
The medical writing market in Germany is experiencing a notable surge due to increased investment in healthcare research and development (R&D). In recent years, the German government has allocated substantial funding to support innovative medical research, which has led to a growing demand for skilled medical writers. This investment is reflected in the €3.5 billion allocated for health research in 2025, indicating a robust commitment to advancing medical knowledge. As pharmaceutical companies and research institutions seek to produce high-quality documentation for regulatory submissions and scientific publications, the need for proficient medical writers becomes paramount. Consequently, this driver is likely to enhance the overall growth of the medical writing market, as organizations strive to meet the rigorous standards set by regulatory bodies.
Emergence of Digital Health Solutions
The rise of digital health solutions is reshaping the landscape of the medical writing market in Germany. With the increasing integration of technology in healthcare, there is a growing need for medical writers to produce content that aligns with digital platforms. This includes writing for mobile health applications, telemedicine platforms, and electronic health records. As the digital health market is projected to reach €10 billion by 2025, the demand for medical writing services that cater to these innovative solutions is likely to grow. Medical writers must adapt to new formats and communication styles, ensuring that content is not only informative but also engaging for users. This evolution presents both challenges and opportunities for the medical writing market, as it navigates the intersection of healthcare and technology.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in Germany is expanding rapidly, which significantly impacts the medical writing market. With the increasing number of biopharmaceutical companies entering the market, there is a heightened demand for specialized medical writing services. In 2025, the biopharmaceutical industry is projected to contribute approximately €15 billion to the German economy, underscoring its importance. This growth necessitates the creation of comprehensive clinical trial documents, regulatory submissions, and scientific publications, all of which require the expertise of medical writers. As biopharmaceutical companies navigate complex regulatory landscapes, the demand for skilled medical writers who can effectively communicate scientific data is likely to rise, thereby driving the medical writing market forward.
Regulatory Compliance and Quality Assurance
Regulatory compliance remains a crucial driver for the medical writing market in Germany. The stringent requirements set forth by regulatory agencies necessitate the production of high-quality documentation that adheres to specific guidelines. In 2025, the European Medicines Agency (EMA) continues to enforce rigorous standards for clinical trial submissions, which places additional pressure on medical writers to ensure accuracy and compliance. This environment creates a demand for experienced medical writers who possess a deep understanding of regulatory frameworks and quality assurance processes. As companies strive to avoid costly delays and rejections in their submissions, the emphasis on regulatory compliance is likely to bolster the medical writing market, as organizations seek to engage skilled professionals to navigate these complexities.
Increased Focus on Patient-Centric Approaches
The medical writing market is witnessing a shift towards patient-centric approaches in healthcare communication. In Germany, healthcare stakeholders are increasingly recognizing the importance of engaging patients in the clinical development process. This trend is reflected in the growing emphasis on creating patient-friendly materials, such as informed consent forms and educational brochures. As a result, medical writers are tasked with the challenge of translating complex medical information into accessible language for patients. This focus on patient engagement is likely to drive demand for medical writing services that prioritize clarity and comprehension, thereby contributing to the growth of the medical writing market. The ability to effectively communicate with patients is becoming a critical component of successful clinical trials and healthcare initiatives.