Supportive Regulatory Environment
The regulatory environment in France plays a crucial role in shaping the human growth hormone market. The French government, along with the European Medicines Agency, has established clear guidelines for the approval and distribution of growth hormone therapies. This supportive framework facilitates the entry of new products into the market, ensuring that patients have access to the latest treatments. Additionally, the reimbursement policies in France encourage healthcare providers to prescribe growth hormone therapies, further stimulating market growth. As regulatory bodies continue to adapt to the evolving landscape of biotechnology, the France human growth hormone market is likely to benefit from increased accessibility and innovation.
Growing Demand for Anti-Aging Treatments
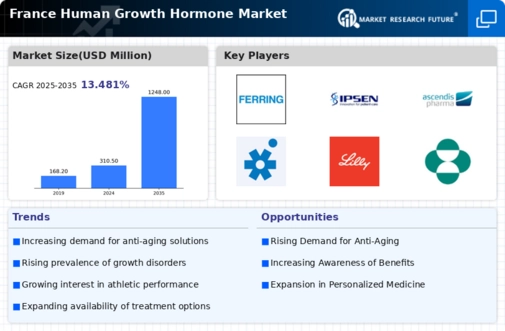
The increasing demand for anti-aging treatments among the aging population in France is emerging as a significant driver for the human growth hormone market. As individuals seek to maintain their vitality and physical appearance, growth hormone therapies are being considered as potential solutions. This trend is particularly pronounced among the middle-aged and older demographics, who are more likely to invest in treatments that promise improved health and longevity. The perception of growth hormone as a means to enhance quality of life may lead to a surge in demand, thereby influencing market dynamics. Consequently, the intersection of aging and wellness is likely to propel the growth of the France human growth hormone market.
Technological Innovations in Hormone Therapy
Technological advancements in hormone therapy are transforming the landscape of the France human growth hormone market. Innovations such as recombinant DNA technology have led to the development of more effective and safer growth hormone products. These advancements not only improve the efficacy of treatments but also reduce the risk of side effects, making them more appealing to both patients and healthcare providers. Furthermore, the introduction of user-friendly delivery systems, such as pre-filled syringes and wearable devices, enhances patient compliance and satisfaction. As these technologies continue to evolve, they are expected to drive market growth by increasing the adoption of growth hormone therapies among patients in France.
Rising Interest in Sports Performance Enhancement
The rising interest in sports performance enhancement is another factor influencing the France human growth hormone market. Athletes and fitness enthusiasts are increasingly exploring growth hormone therapies as a means to improve physical performance, recovery, and muscle mass. This trend is fueled by the competitive nature of sports and the desire for optimal performance. While the use of growth hormones in professional sports is heavily regulated, the interest among amateur athletes remains strong. As awareness of the potential benefits of growth hormone therapies grows, it is likely to create a niche market segment within the broader France human growth hormone market, attracting both consumers and manufacturers.
Increasing Prevalence of Growth Hormone Deficiency
The rising prevalence of growth hormone deficiency in France is a notable driver for the France human growth hormone market. Recent studies indicate that approximately 1 in 4,000 children may be affected by this condition, leading to significant demand for growth hormone therapies. As awareness of the symptoms and long-term impacts of untreated deficiencies grows, healthcare providers are increasingly diagnosing and treating affected individuals. This trend is further supported by the French healthcare system's commitment to providing necessary treatments, which enhances patient access to growth hormone therapies. Consequently, the increasing prevalence of growth hormone deficiency is likely to propel the market forward, as more patients seek effective solutions to manage their conditions.