Growing Awareness and Education
There is a notable increase in awareness and education regarding heart tumors in France, which is positively influencing the heart tumor market. Public health campaigns and educational initiatives aimed at both healthcare professionals and the general population are helping to demystify heart tumors. As awareness grows, patients are more likely to seek medical attention for symptoms that may indicate a heart tumor, leading to earlier diagnosis and treatment. This shift in patient behavior is likely to drive demand for diagnostic services and therapeutic interventions within the heart tumor market. Additionally, healthcare providers are becoming more knowledgeable about the latest advancements in treatment options, which may further enhance patient care. The emphasis on education and awareness is expected to contribute to a more informed patient population, ultimately benefiting the heart tumor market.
Rising Incidence of Heart Tumors
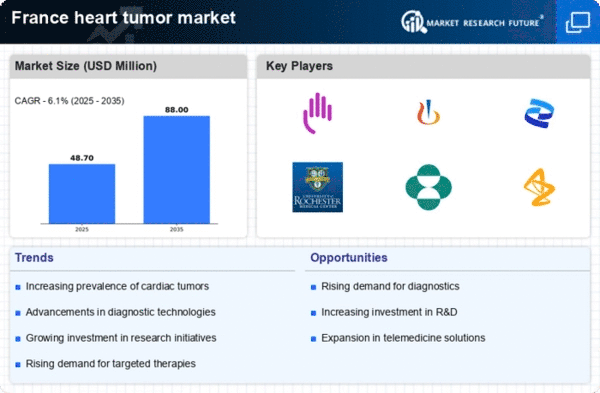
The heart tumor market in France is experiencing growth due to an increasing incidence of heart tumors. Recent data indicates that the prevalence of primary cardiac tumors is on the rise, with estimates suggesting that around 0.02 to 0.03 cases per 100,000 individuals are diagnosed annually. This trend is likely to drive demand for diagnostic and therapeutic solutions within the heart tumor market. As awareness of heart tumors increases, healthcare providers are more frequently identifying these conditions, leading to a greater need for specialized treatments. The growing patient population necessitates advancements in medical technology and treatment options, thereby propelling the heart tumor market forward. Furthermore, the aging population in France, which is more susceptible to various forms of cancer, may contribute to this upward trend, indicating a sustained demand for innovative solutions in the heart tumor market.
Advancements in Treatment Modalities
Innovations in treatment modalities are significantly impacting the heart tumor market in France. The development of minimally invasive surgical techniques and targeted therapies has transformed the landscape of cardiac oncology. For instance, the introduction of robotic-assisted surgeries has improved patient outcomes and reduced recovery times, making these procedures more appealing to both patients and healthcare providers. Additionally, the integration of novel pharmacological agents, such as immunotherapies, is expanding treatment options for patients with heart tumors. As these advancements continue to emerge, they are likely to enhance the efficacy of treatments, thereby increasing the overall market size. The heart tumor market is expected to benefit from ongoing research and development efforts, which may lead to the introduction of new therapies that could potentially improve survival rates and quality of life for patients.
Increased Funding for Cancer Research
The heart tumor market in France is poised for growth due to increased funding for cancer research. Government and private sector investments in oncology research have surged, with funding allocations reaching approximately €1 billion annually. This financial support is directed towards understanding the biology of heart tumors and developing novel therapeutic strategies. As research initiatives expand, they are likely to yield breakthroughs that can be translated into clinical practice, thereby enhancing treatment options available in the heart tumor market. Furthermore, collaborations between academic institutions and pharmaceutical companies are fostering innovation, which may lead to the discovery of new biomarkers and targeted therapies. This influx of funding not only supports the development of new treatments but also encourages clinical trials, which are essential for validating the efficacy of emerging therapies in the heart tumor market.
Regulatory Environment Favoring Innovation
The regulatory environment in France is becoming increasingly favorable for innovation in the heart tumor market. Recent reforms aimed at expediting the approval process for new therapies and medical devices are encouraging companies to invest in research and development. The French National Agency for the Safety of Medicines and Health Products (ANSM) has implemented measures to streamline regulatory pathways, which may lead to faster access to novel treatments for patients. This supportive regulatory framework is likely to stimulate competition among pharmaceutical companies, driving innovation in the heart tumor market. As new therapies receive approval, they can quickly enter the market, providing patients with more options for treatment. The proactive stance of regulatory bodies in France may thus play a crucial role in shaping the future landscape of the heart tumor market.