Advancements in Therapeutic Options
The Exocrine Pancreatic Insufficiency Therapeutics Market is witnessing significant advancements in therapeutic options, which serve as a crucial market driver. The introduction of novel enzyme replacement therapies and innovative formulations has expanded treatment choices for patients suffering from EPI. For instance, recent developments in enteric-coated formulations have improved the bioavailability of pancreatic enzymes, leading to better patient outcomes. Market data indicates that the enzyme replacement therapy segment is projected to grow at a compound annual growth rate (CAGR) of over 6% in the coming years. These advancements not only enhance the efficacy of treatments but also improve patient adherence, as more effective therapies can lead to better management of symptoms. As a result, the market is likely to experience robust growth fueled by these innovations.
Growing Focus on Patient-Centric Care
The shift towards patient-centric care is emerging as a significant driver for the Exocrine Pancreatic Insufficiency Therapeutics Market. Healthcare providers are increasingly prioritizing individualized treatment plans that cater to the specific needs of EPI patients. This approach not only enhances patient satisfaction but also improves treatment adherence and outcomes. The integration of patient feedback into therapeutic development processes is becoming more common, leading to the creation of therapies that align closely with patient preferences. Furthermore, the emphasis on quality of life in treatment decisions is likely to influence the types of therapies developed and marketed. As the healthcare landscape evolves to embrace patient-centric models, the demand for tailored therapeutic solutions for EPI is expected to grow, thereby propelling market expansion.
Rising Awareness and Diagnosis of EPI
Increased awareness and improved diagnostic capabilities are significantly influencing the Exocrine Pancreatic Insufficiency Therapeutics Market. Healthcare professionals are becoming more adept at recognizing the symptoms associated with EPI, leading to earlier diagnosis and treatment initiation. This heightened awareness is partly due to educational initiatives and the dissemination of information regarding EPI's impact on quality of life. Market Research Future indicates that the number of diagnosed cases of EPI has risen, with estimates suggesting that around 50% of patients with chronic pancreatitis may be undiagnosed. As more patients receive appropriate diagnoses, the demand for therapeutic options is expected to surge, thereby driving market growth. This trend underscores the importance of continued education and awareness efforts in enhancing patient outcomes and expanding the therapeutic market.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supporting the development of innovative therapies for exocrine pancreatic insufficiency, which serves as a vital driver for the Exocrine Pancreatic Insufficiency Therapeutics Market. Initiatives aimed at expediting the approval process for new treatments are encouraging pharmaceutical companies to invest in research and development. For instance, the designation of orphan drug status for certain EPI therapies can provide incentives such as tax credits and market exclusivity, fostering innovation. This regulatory support is crucial in a market characterized by a relatively small patient population, as it encourages the development of specialized treatments that may otherwise be deemed unprofitable. As more therapies gain regulatory approval, the market is likely to witness an influx of new products, enhancing treatment options for patients and driving overall market growth.
Increasing Prevalence of Exocrine Pancreatic Insufficiency
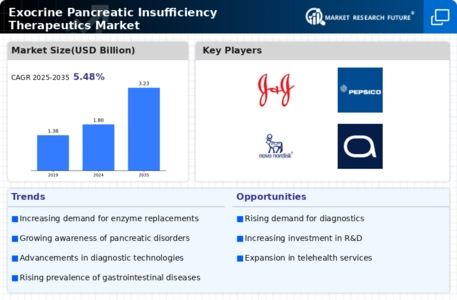
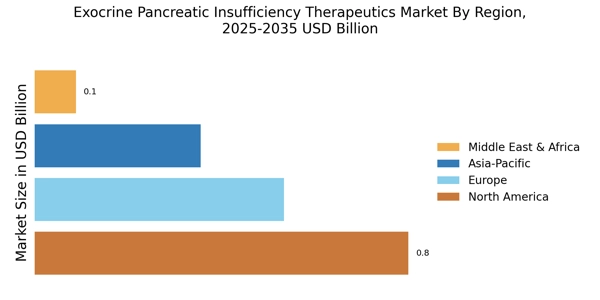
The rising incidence of exocrine pancreatic insufficiency (EPI) is a primary driver for the Exocrine Pancreatic Insufficiency Therapeutics Market. Conditions such as cystic fibrosis, chronic pancreatitis, and pancreatic cancer contribute to the growing number of patients diagnosed with EPI. Recent estimates suggest that approximately 1 in 10 individuals with chronic pancreatitis may develop EPI, indicating a substantial patient population requiring therapeutic interventions. This increasing prevalence necessitates the development and availability of effective treatments, thereby propelling market growth. As healthcare systems recognize the need for specialized therapies, investments in research and development are likely to increase, further enhancing the therapeutic landscape for EPI. Consequently, the demand for innovative solutions tailored to manage EPI effectively is expected to rise, driving the market forward.