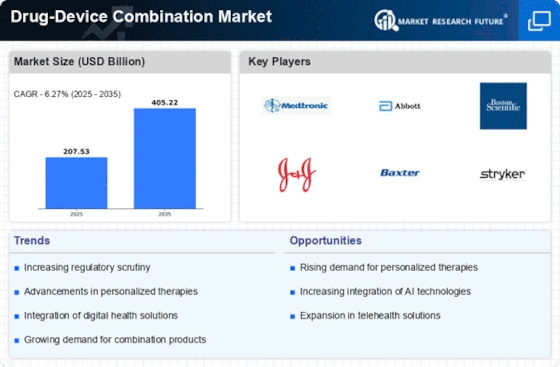
Top Industry Leaders in the Drug Device Combination Market
 Latest Drug Device Combination Companies Update
Latest Drug Device Combination Companies Update
-
Oct 2023 The diversified manufacturer, 3M Co., had its shares rise more than 5% because it raised its full-year adjusted profit projection and reported strong quarterly earnings. The company was able to counteract poor demand with price increases and expense reductions. In response to rising labor and raw material costs, 3M increased pricing and reduced staff as the market for consumer electronics declined. Further price increases in the face of growing living expenses may prove difficult, given that American households are deferring large purchases and reducing discretionary spending due to an uncertain economic future. Although the company's efforts to reduce costs allowed it to surpass projections, 3M is confronted with a sluggish market for consumer electronics, particularly in China and Europe.
-
Sep 2023 The latest generation WATCHMAN FLXTM Pro Left Atrial Appendage Closure (LAAC) Device has been approved by the US Food and Drug Administration, according to a statement released by Boston Scientific Corporation. With a polymer coating, visualization markers, and a broader size matrix to treat a wider range of patients, the device is designed to enhance the procedural effectiveness and security of the WATCHMAN technology, which has been shown to minimize stroke risk among individuals with non-valvular atrial fibrillation that require an alternative to oral anticoagulation therapy. Based on the well-established safety and procedural efficacy of the WATCHMAN FLXTM LAAC device—which was approved in July 2020 and has been utilized in approximately 190,000 of the greater than 300,000 WATCHMAN procedures that have been safely done to date—the WATCHMAN FLX Pro device globally.
List of Drug Device Combination Companies in the market
- 3M
- Abbott Laboratories
- AlloSource
- Biomet Orthopedics Inc
- Biometrix Medical
- Biotronik
- Boston Scientific Corporation
- R. BARD Inc
- Cook Critical Care Inc
- Covidien Ltd