Research Methodology Covid Testing Kit Market
Executive Summary
The purpose of this research report is to examine the global market for Covid-testing kits. This research utilizes several research techniques and approaches to provide an accurate and detailed analysis of the market. By looking at various primary and secondary sources, the research provides a comprehensive market overview and analyzes the current state of the Covid-testing kits market. Additionally, this research report also calculates the potential growth opportunities for this market in the near future.
Introduction
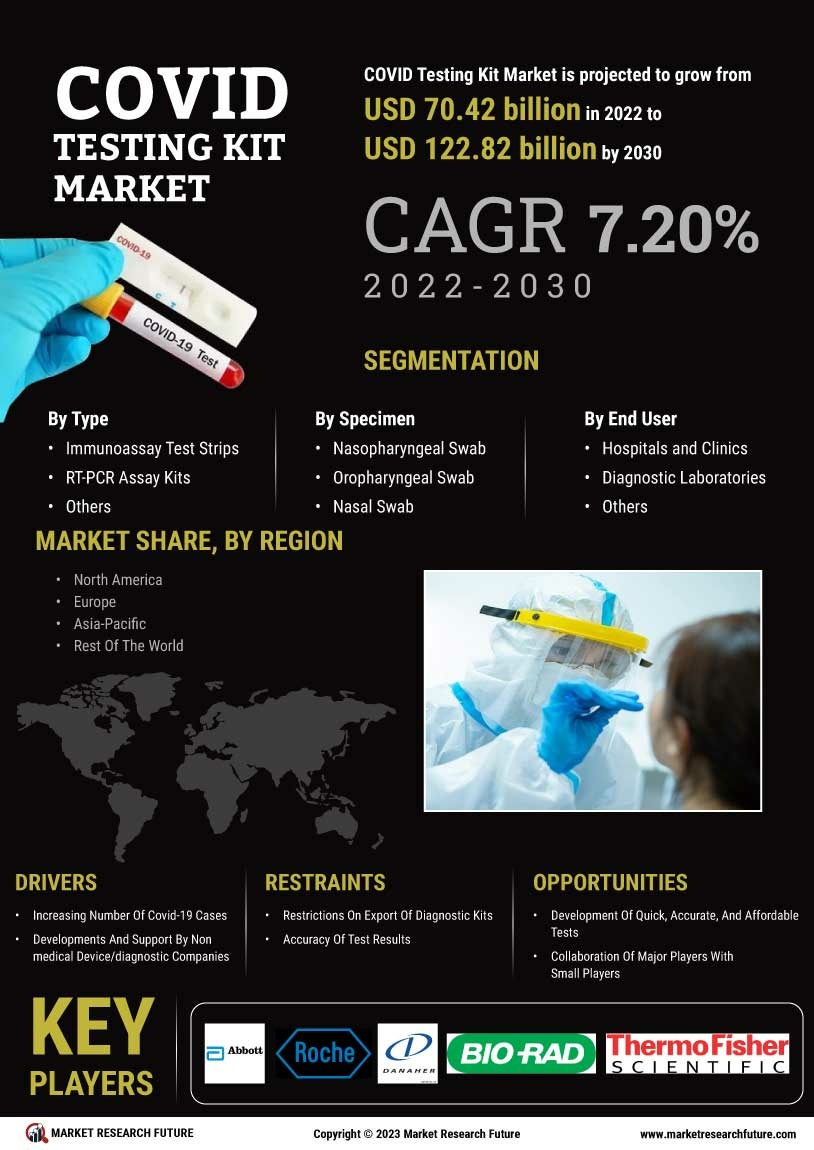
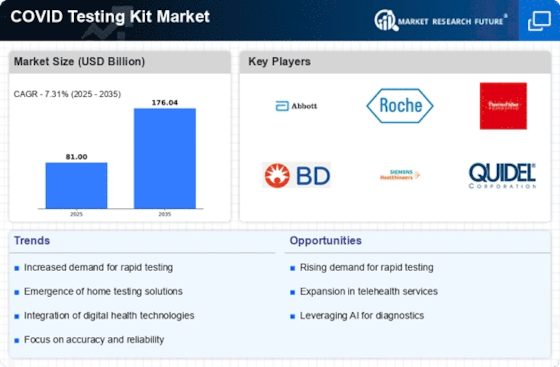
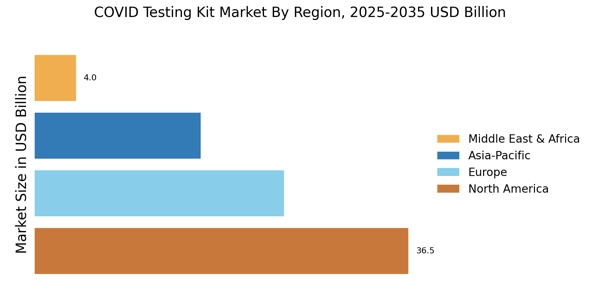
The Covid-testing kits market experiences a huge jump in sales due to the ongoing pandemic and the need for quick and accurate diagnosis of Covid-19 infections. As such, the market has been experiencing a large number of innovations, with each company offering a variety of features and capabilities. This paper seeks to analyze the global Covid-testing kits market and understand its potential for growth over the next few years. This research report studies the market from both a quantitative and qualitative perspective.
Research Objectives
The primary objectives of this research report are to:
- Identify and analyze the major trends in the Covid-testing kits market.
- Understand the current status of the market and its future trends.
- Investigate the potential growth opportunities of the market.
- Study the major players in the market and analyze their positioning.
Research Questions
- What are the major trends in the Covid-testing kits market?
- What is the current status of the market?
- What are the major growth opportunities in the market?
- Who are the major players in the market and what is their positioning?
Research Methodology
The published research report focuses on the use of both primary and secondary research techniques to provide an accurate and comprehensive analysis of the global Covid-testing kits market. Primary research techniques involve conducting interviews with leading industry experts, conducting surveys with industry participants, and researching the industry-specific factors driving the growth of the Covid-testing market. Secondary research methods allow for the identification of reliable and up-to-date sources of information, such as industry reports, academic journals, and other such sources. Additionally, the paper uses quantitative and qualitative data from reliable sources such as the United Nations Economic Commission, World Bank reports, consulting reports, and other such sources.
Data Collection
The primary sources of data used in this report include industry reports, government reports, and online surveys. The industry reports mainly focus on the Covid-testing market and provide an overview of the market size, trends, and other relevant information. Government reports that will be used for this research include the United Nations Economic Commission for Europe (UNECE), World Bank, the International Monetary Fund (IMF), and other such sources. The online surveys are conducted amongst the industry participants and provide an understanding of the current status of the Covid-testing market.
Analysis
The analysis phase of the research paper entails the use of both quantitative and qualitative methods. The quantitative analysis phase involves the use of various statistical tools such as trend analysis, market sizing, and forecasting. This phase allows for the identification of the various opportunities and threats faced by the market, as well as the current trends and prospects. The qualitative analysis phase involves the analysis of both primary and secondary data sources and provides a comprehensive understanding of the market.
Findings
The findings of the research paper include a comprehensive overview of the market, an analysis of the major trends, an understanding of the current market status, and the potential growth opportunities available in the near future. Additionally, the major players in the market and their positioning are identified and analyzed.
Conclusion
The global Covid-testing kits market has experienced a huge jump in sales due to the ongoing pandemic and the need for quick and accurate diagnosis of Covid-19 infections. This research report has used some primary and secondary research techniques to provide a comprehensive overview of the market and analyze the potential growth opportunities available. The report also identifies the major trends, the current market status, and the major players in the market. A detailed analysis of this market is expected to help identify further opportunities for businesses and investors.