Rising Prevalence of Cardiovascular Conditions
The Clot Management Device Market is significantly influenced by the rising prevalence of cardiovascular conditions worldwide. As lifestyle-related diseases become more common, the demand for effective clot management solutions is increasing. According to recent statistics, cardiovascular diseases account for a substantial percentage of global mortality, prompting healthcare systems to prioritize the development and deployment of advanced clot management devices. This trend is further supported by an aging population, which is more susceptible to such conditions. Consequently, healthcare providers are investing in innovative technologies to address this growing need. The Clot Management Device Market is expected to expand as a result, with projections indicating a potential increase in market size driven by the urgent requirement for effective treatment options for patients suffering from cardiovascular ailments.
Regulatory Influence on Clot Management Devices
Regulatory frameworks play a crucial role in shaping the Clot Management Device Market. Stringent regulations imposed by health authorities ensure that devices meet safety and efficacy standards before they reach the market. This regulatory oversight not only protects patients but also fosters innovation by encouraging manufacturers to invest in research and development. In recent years, there has been a trend towards expedited approval processes for breakthrough devices, which could potentially accelerate the introduction of novel clot management solutions. As a result, companies are increasingly focusing on compliance with regulatory requirements to gain a competitive edge. The evolving regulatory landscape is likely to influence market dynamics, as manufacturers adapt their strategies to align with these standards, ultimately impacting the growth trajectory of the Clot Management Device Market.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a pivotal driver for the Clot Management Device Market. Governments and private entities are recognizing the importance of enhancing healthcare facilities to improve patient care. This investment often translates into the acquisition of advanced medical devices, including those used for clot management. As hospitals and clinics upgrade their equipment, the demand for state-of-the-art clot management devices is likely to rise. Furthermore, the establishment of specialized cardiovascular centers is becoming more prevalent, which could further stimulate the market. Enhanced healthcare infrastructure not only facilitates better access to treatment but also encourages the adoption of innovative technologies. As a result, the Clot Management Device Market is poised for growth, reflecting the broader trend of healthcare modernization and the commitment to improving patient outcomes.
Growing Awareness and Education on Clot Management
The Clot Management Device Market is benefiting from growing awareness and education regarding clot management among both healthcare professionals and patients. Increased educational initiatives and training programs are equipping medical personnel with the knowledge necessary to utilize advanced clot management devices effectively. Additionally, public awareness campaigns are informing patients about the risks associated with clot formation and the importance of timely intervention. This heightened awareness is likely to lead to an increase in demand for clot management solutions, as patients become more proactive in seeking treatment. Moreover, as healthcare providers emphasize the importance of early detection and intervention, the market for clot management devices is expected to expand. The Clot Management Device Market is thus positioned to grow, driven by a more informed patient population and a well-trained healthcare workforce.
Technological Advancements in Clot Management Devices
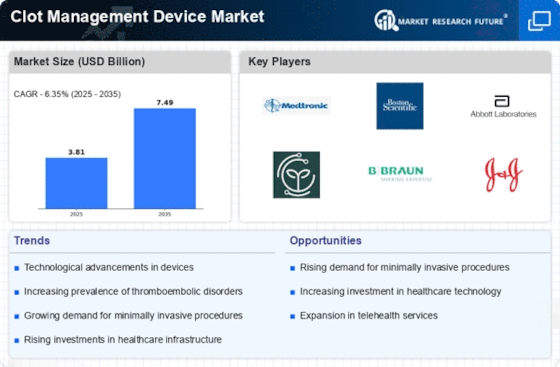
The Clot Management Device Market is experiencing a notable transformation due to rapid technological advancements. Innovations such as minimally invasive techniques and enhanced imaging technologies are revolutionizing the way clot management is approached. For instance, devices equipped with advanced ultrasound and fluoroscopy capabilities are improving the accuracy of clot detection and removal. The market is projected to grow at a compound annual growth rate of approximately 6.5% over the next few years, driven by these advancements. Furthermore, the integration of artificial intelligence in device functionality is expected to enhance decision-making processes during procedures, thereby improving patient outcomes. As healthcare providers increasingly adopt these cutting-edge technologies, the Clot Management Device Market is likely to witness a surge in demand, reflecting a shift towards more efficient and effective treatment options.