Market Trends
Key Emerging Trends in the Clinical Trial Imaging Market
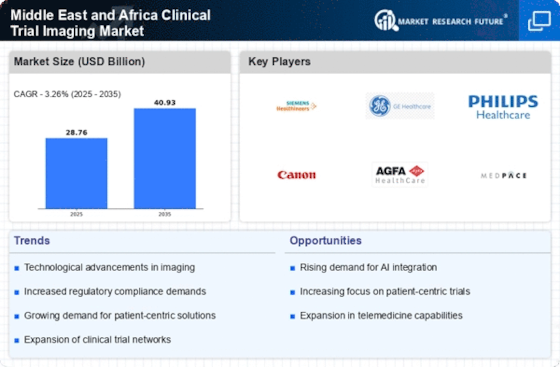
The Middle East and Africa clinical trial imaging market is growing as more clinical studies are done there. Imaging services are needed more as the pharmaceutical and biotechnology sectors develop. These services help evaluate new medications and medical interventions. Imaging technology advancements impact industry developments. PET, MRI, and CT are prominent imaging modalities. These imaging methods are cutting-edge. These imaging modalities provide researchers more complete data for clinical trial evaluations. Growing precision medicine emphasis makes the industry more vulnerable to various affects. Imaging helps identify patient subgroups that may benefit from various drugs in this kind of therapy. Due to this tendency, advanced imaging tools that can assess the efficacy of focused pharmacological therapies are in demand. AI is becoming increasingly popular in clinical trial imaging. Artificial intelligence algorithms are utilized for image processing, interpretation, and biomarker identification to streamline the imaging process, reduce human error, and improve clinical trials. It's becoming clear that clinical trials are diminishing imaging services. To achieve uniformity and consistency in imaging data assessment across several trial locations, centralized imaging core laboratories are being used. This will increase data quality and simplify regulatory form submission. Clinical trial imaging is driven by the current increase in cancer clinical research. Due to escalating cancer rates in the Middle East and Africa, novel imaging tools to monitor tumor response to treatment are being developed and tested. This is because more individuals are getting that sickness. This has increased the demand for cancer clinical trial imaging services. Remote imaging monitoring is improving market dynamics. Real-time remote imaging data monitoring and analysis are now feasible because of technological advances. This may improve clinical trial operations and reduce travels to the experiment site. This is crucial for global clinical studies. Imaging service providers are joining hands with pharmaceutical firms, as well as research institutes to drive clinical trial imaging innovation. New technologies are being developed jointly. Strategic collaborations are being formed to improve imaging capabilities, increase services, and accelerate the development of breakthrough imaging technologies for clinical trials. The increased relevance of regulatory compliance and standardization is affecting market trends. Regulatory authorities are emphasizing imaging data integrity, so standardized imaging procedures, quality assurance methods, and regulatory criteria are being used to ensure clinical trial validity. This ensures clinical study results are reliable. Despite favorable trends, skills and infrastructure remain a barrier. Training, technological infrastructure, and collaborations are being used to solve these issues. These initiatives aim to equip professionals to handle complicated imaging technology in clinical trials.

















Leave a Comment