Growing Focus on Patient-Centric Trials
The Clinical Data Management System Market is increasingly influenced by the growing focus on patient-centric trials. As the industry shifts towards models that prioritize patient engagement and experience, data management systems must adapt to capture diverse data types, including patient-reported outcomes. This trend is reflected in the rising number of decentralized clinical trials, which require flexible data management solutions that can accommodate various data sources. Organizations are recognizing the importance of integrating patient feedback into their data management processes, which enhances the overall quality of clinical trial data. Consequently, the demand for clinical data management systems that support patient-centric approaches is likely to increase, as stakeholders aim to improve trial outcomes and patient satisfaction.
Regulatory Compliance and Standardization
Regulatory compliance remains a critical driver in the Clinical Data Management System Market. As regulatory bodies impose stringent guidelines for clinical trials, organizations are compelled to adopt data management systems that ensure adherence to these regulations. The need for compliance with standards such as Good Clinical Practice (GCP) and the International Conference on Harmonisation (ICH) guidelines is driving the adoption of sophisticated data management solutions. Furthermore, the increasing scrutiny from regulatory authorities necessitates the implementation of systems that can provide comprehensive audit trails and data security. This trend indicates that organizations are likely to invest in clinical data management systems that not only facilitate compliance but also enhance the overall quality of clinical trial data.
Rising Demand for Efficient Data Management
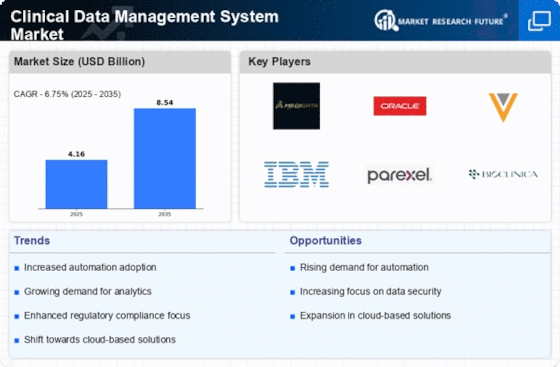
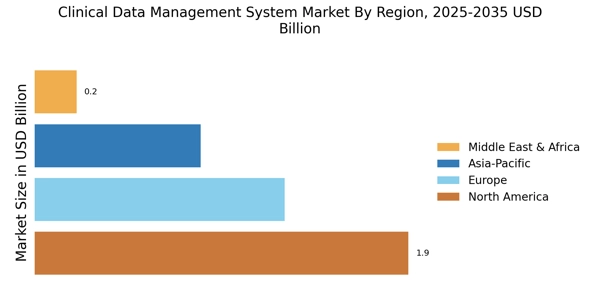
The Clinical Data Management System Market is experiencing a notable increase in demand for efficient data management solutions. As clinical trials become more complex, the need for robust data management systems that can handle large volumes of data is paramount. According to recent estimates, the market is projected to grow at a compound annual growth rate of approximately 12% over the next five years. This growth is driven by the increasing number of clinical trials and the necessity for accurate data collection and analysis. Organizations are seeking systems that not only streamline data management processes but also enhance data integrity and compliance with regulatory standards. Consequently, the demand for advanced clinical data management systems is likely to rise, as stakeholders prioritize efficiency and accuracy in their operations.
Technological Advancements in Data Management
Technological advancements are significantly influencing the Clinical Data Management System Market. Innovations such as cloud computing, big data analytics, and machine learning are transforming how clinical data is managed and analyzed. These technologies enable organizations to process vast amounts of data more efficiently and derive actionable insights. For instance, the integration of cloud-based solutions allows for real-time data access and collaboration among stakeholders, which is essential for modern clinical trials. As a result, the market is witnessing a shift towards more sophisticated data management systems that leverage these technologies. The potential for improved data accuracy and faster decision-making processes is likely to drive further investment in advanced clinical data management solutions.
Increased Investment in Research and Development
Investment in research and development is a significant driver of the Clinical Data Management System Market. As pharmaceutical and biotechnology companies allocate more resources to R&D, the need for efficient data management systems becomes increasingly apparent. The rising costs associated with drug development and the pressure to bring new therapies to market quickly necessitate the implementation of robust data management solutions. Organizations are seeking systems that can streamline data collection, enhance data quality, and facilitate regulatory submissions. This trend suggests that the market for clinical data management systems will continue to expand, as companies prioritize investments in technologies that support their R&D efforts and improve overall operational efficiency.