Advancements in Pharmaceutical Research
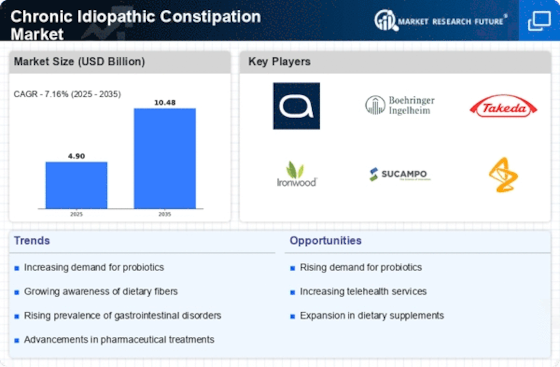
Innovations in pharmaceutical research are significantly influencing the Chronic Idiopathic Constipation Market. Recent developments in drug formulations and delivery systems have led to the introduction of novel therapies that target the underlying mechanisms of constipation. For instance, medications that enhance gastrointestinal motility or modify gut microbiota are gaining traction. The market is projected to witness a compound annual growth rate of around 5% over the next few years, driven by these advancements. Furthermore, ongoing clinical trials and research initiatives are likely to yield new treatment options, thereby expanding the therapeutic landscape and enhancing patient outcomes in the Chronic Idiopathic Constipation Market.
Integration of Digital Health Solutions
The integration of digital health solutions is emerging as a transformative driver in the Chronic Idiopathic Constipation Market. Telehealth services and mobile health applications are facilitating remote consultations and personalized treatment plans for patients suffering from chronic idiopathic constipation. These technologies not only enhance patient engagement but also improve access to healthcare professionals, particularly in underserved areas. As digital health continues to evolve, it is expected to play a crucial role in monitoring patient progress and adherence to treatment regimens. This trend may lead to improved outcomes and increased market penetration for digital solutions within the Chronic Idiopathic Constipation Market.
Rising Demand for Over-the-Counter Solutions
The demand for over-the-counter (OTC) solutions for chronic idiopathic constipation is on the rise, significantly impacting the Chronic Idiopathic Constipation Market. Consumers are increasingly seeking accessible and convenient treatment options that do not require a prescription. This trend is particularly evident among younger populations who prefer self-management of their health conditions. The OTC segment is expected to grow as manufacturers introduce a variety of laxatives and dietary supplements aimed at alleviating constipation symptoms. This shift towards self-care and the availability of effective OTC products are likely to drive market expansion within the Chronic Idiopathic Constipation Market.
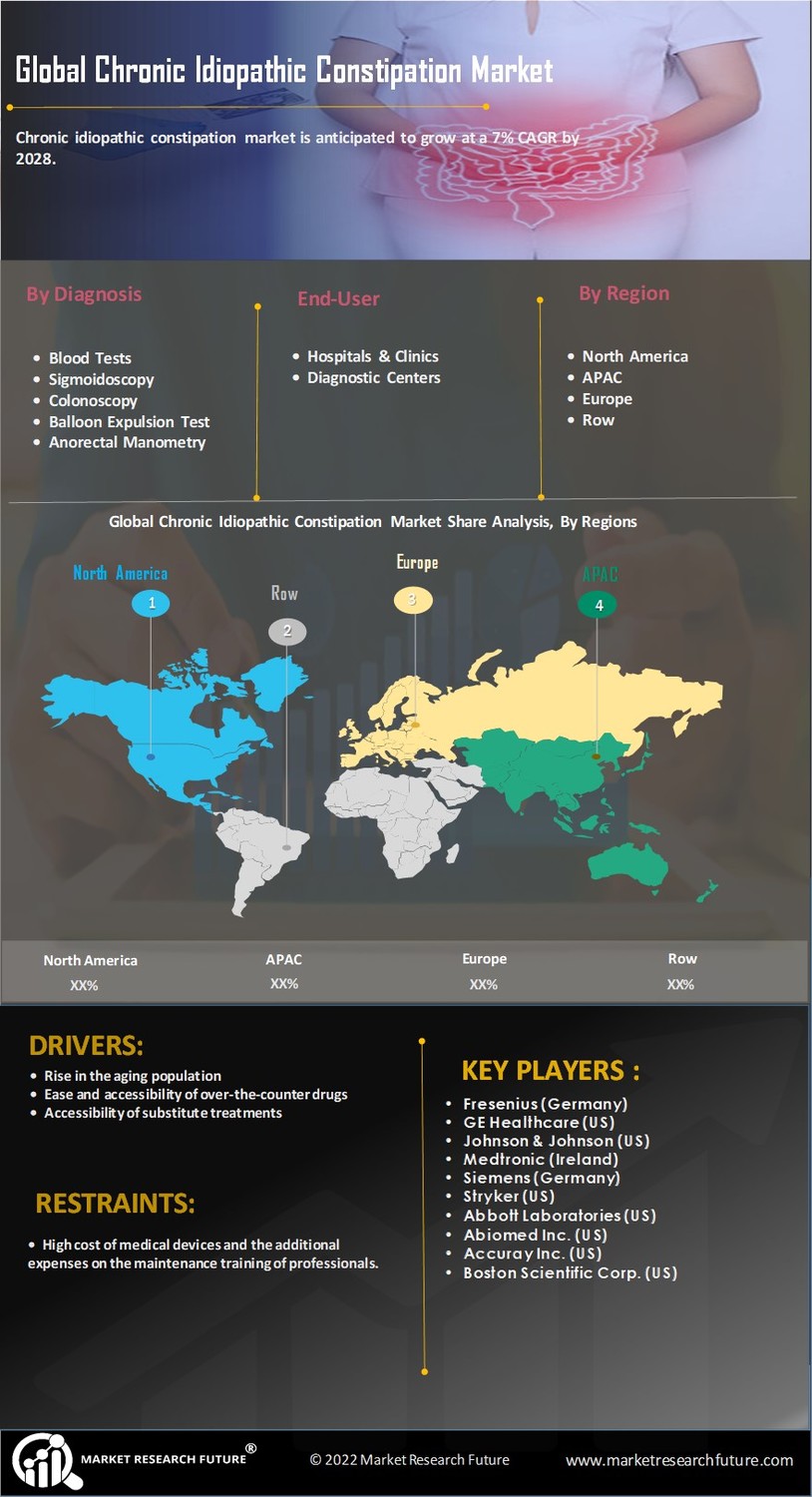
Increasing Prevalence of Chronic Idiopathic Constipation
The rising incidence of chronic idiopathic constipation is a pivotal driver for the Chronic Idiopathic Constipation Market. Studies indicate that approximately 14% of the population experiences this condition, with a notable increase in prevalence among older adults. This demographic shift, coupled with lifestyle changes such as poor dietary habits and sedentary behavior, contributes to a growing patient population. As awareness of gastrointestinal health expands, more individuals seek medical advice, leading to increased demand for effective treatments. The Chronic Idiopathic Constipation Market is thus poised for growth, as healthcare providers and pharmaceutical companies respond to this rising need with innovative therapies and solutions.
Growing Awareness and Education on Gastrointestinal Health
The increasing focus on gastrointestinal health education is a crucial driver for the Chronic Idiopathic Constipation Market. Public health campaigns and educational initiatives are raising awareness about the symptoms and treatment options available for chronic idiopathic constipation. This heightened awareness encourages individuals to seek medical consultation, thereby increasing diagnosis rates. As a result, healthcare providers are more likely to recommend appropriate therapies, contributing to market growth. Additionally, the integration of educational resources into healthcare settings fosters a better understanding of preventive measures, which may further influence the Chronic Idiopathic Constipation Market positively.