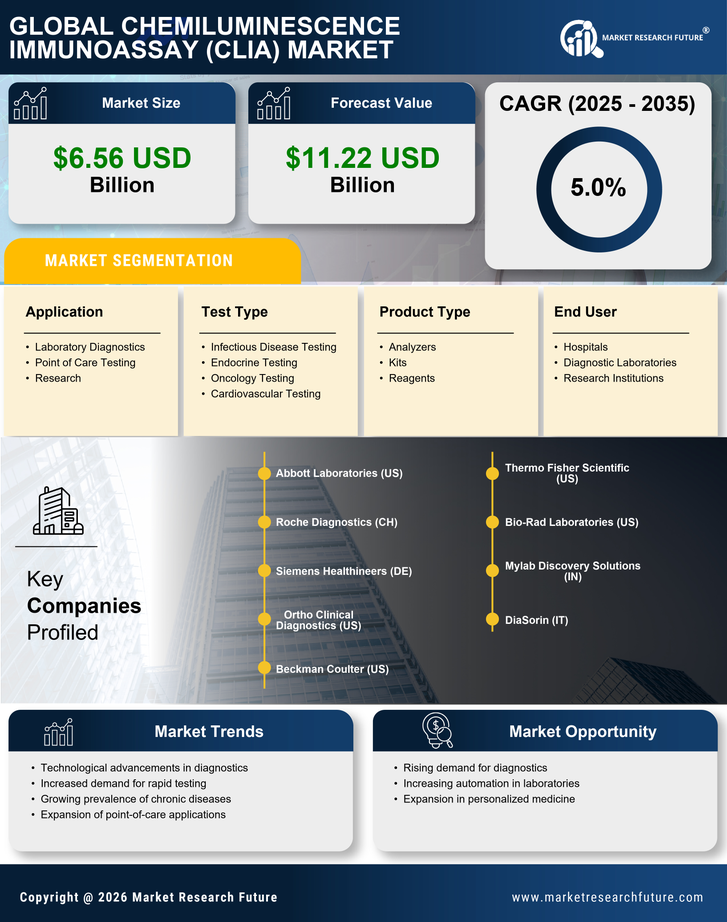
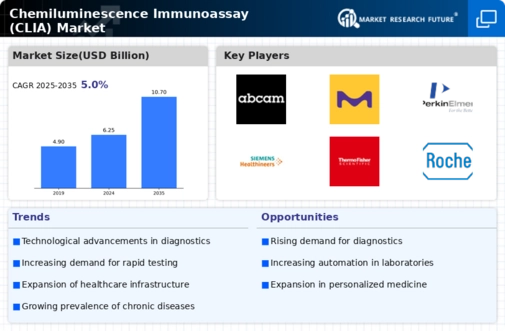
Q2 2024: Siemens Healthineers Launches Atellica CI Analyzer for Chemiluminescence Immunoassays Siemens Healthineers announced the commercial launch of its Atellica CI Analyzer, a new chemiluminescence immunoassays system designed for mid-volume laboratories, expanding its Atellica portfolio and aiming to improve workflow efficiency and diagnostic accuracy.
Q2 2024: BioMérieux Receives CE Mark for VIDAS KUBE Chemiluminescence Immunoassay Market Platform BioMérieux announced that its VIDAS KUBE, a next-generation chemiluminescence immunoassays platform, received CE marking, allowing commercialization in the European Union and supporting the company's expansion in automated diagnostics.
Q3 2024: Abbott Launches New Chemiluminescent SARS-CoV-2 Antigen Test in Europe Abbott announced the launch of a new chemiluminescent immunoassay-based SARS-CoV-2 antigen test for use on its ARCHITECT and Alinity i platforms, expanding its COVID-19 diagnostics portfolio in the European market.
Q3 2024: Roche Receives FDA Clearance for Elecsys Anti-Müllerian Hormone Plus Immunoassay Roche announced that the U.S. FDA cleared its Elecsys Anti-Müllerian Hormone Plus chemiluminescence immunoassays, enabling broader use in fertility testing and reproductive health diagnostics in the United States.
Q4 2024: Mindray Opens New Chemiluminescence Immunoassay Market Manufacturing Facility in Singapore Mindray, a leading medical device manufacturer, inaugurated a new production facility in Singapore dedicated to chemiluminescence immunoassays analyzers, aiming to strengthen its supply chain and meet growing global demand.
Q1 2025: Thermo Fisher Scientific Acquires ImmunoTech Diagnostics to Expand Chemiluminescence Immunoassay Market Portfolio Thermo Fisher Scientific completed the acquisition of ImmunoTech Diagnostics, a company specializing in chemiluminescence immunoassays reagents and systems, to enhance its offerings in clinical diagnostics.
Q1 2025: Beckman Coulter Launches Access SARS-CoV-2 IgG II Chemiluminescent Immunoassay in Japan Beckman Coulter announced the launch of its Access SARS-CoV-2 IgG II chemiluminescent immunoassay in Japan, following regulatory approval, to support COVID-19 antibody testing in clinical laboratories.
Q2 2025: Siemens Healthineers and Sysmex Announce Strategic Partnership for Chemiluminescence Immunoassay Market Development Siemens Healthineers and Sysmex Corporation entered a strategic partnership to co-develop next-generation chemiluminescence immunoassays platforms, aiming to accelerate innovation and expand their global diagnostics footprint.
Q2 2025: FDA Approves DiaSorin LIAISON XL Meningitis/Encephalitis Chemiluminescence Immunoassay Market Panel The U.S. FDA approved DiaSorin's LIAISON XL Meningitis/Encephalitis Panel, a multiplex chemiluminescence immunoassay, for use in clinical laboratories to aid in the rapid diagnosis of central nervous system infections.
Q2 2025: QuidelOrtho Secures $100 Million Contract for Chemiluminescence Immunoassay Market Systems with Major U.S. Hospital Network QuidelOrtho announced it has secured a $100 million multi-year contract to supply chemiluminescence immunoassay analyzers and reagents to a leading U.S. hospital network, strengthening its position in the clinical diagnostics market.