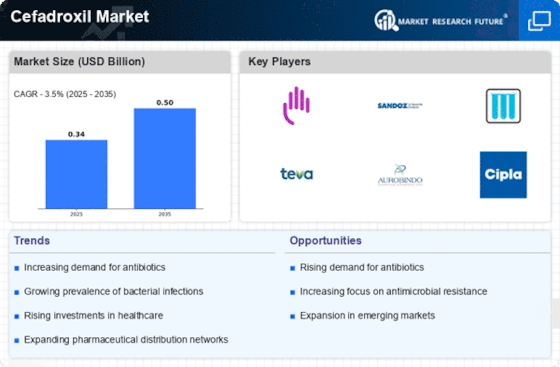
Growing Geriatric Population
The Cefadroxil Market is significantly impacted by the growing geriatric population, which is more susceptible to bacterial infections. As the global population ages, the incidence of infections among older adults is likely to rise, leading to an increased demand for effective antibiotics like cefadroxil. Data indicates that older adults often have comorbidities that complicate treatment, making the need for reliable antibiotic options even more critical. Consequently, healthcare providers are expected to prescribe cefadroxil more frequently to this demographic, thereby driving growth in the Cefadroxil Market. This trend underscores the importance of cefadroxil in addressing the unique healthcare needs of the aging population.
Expansion of Healthcare Infrastructure
The Cefadroxil Market is poised for growth due to the expansion of healthcare infrastructure in various regions. As healthcare facilities increase in number and capacity, access to essential medications, including cefadroxil, improves significantly. This expansion is particularly evident in developing regions, where investments in healthcare are on the rise. Data suggests that as more healthcare facilities become equipped to diagnose and treat bacterial infections, the demand for cefadroxil is likely to increase. Furthermore, the establishment of new hospitals and clinics is expected to facilitate the availability of cefadroxil, thereby enhancing its market penetration and overall growth in the Cefadroxil Market.
Rising Awareness of Antibiotic Stewardship
The Cefadroxil Market is influenced by the increasing awareness surrounding antibiotic stewardship programs. These initiatives aim to promote the responsible use of antibiotics to combat the growing threat of antibiotic resistance. As healthcare institutions implement these programs, there is a heightened focus on prescribing effective antibiotics, such as cefadroxil, for appropriate infections. This trend is supported by data indicating that hospitals adopting antibiotic stewardship have reported a decrease in antibiotic resistance rates. Consequently, the emphasis on judicious antibiotic use is likely to bolster the demand for cefadroxil, as it is recognized for its efficacy against specific bacterial infections, thus enhancing its market presence.
Increasing Incidence of Bacterial Infections
The Cefadroxil Market is experiencing growth due to the rising incidence of bacterial infections across various demographics. As antibiotic-resistant strains of bacteria become more prevalent, the demand for effective antibiotics like cefadroxil is likely to increase. According to recent data, bacterial infections account for a significant percentage of hospital admissions, necessitating the use of antibiotics for treatment. This trend suggests that healthcare providers are increasingly relying on cefadroxil as a first-line treatment option, thereby driving market expansion. Furthermore, the growing awareness among patients regarding the importance of timely treatment for bacterial infections is expected to contribute to the rising demand for cefadroxil, reinforcing its position in the Cefadroxil Market.
Technological Advancements in Drug Formulation
The Cefadroxil Market is benefiting from technological advancements in drug formulation and delivery systems. Innovations in pharmaceutical technology have led to the development of more effective and patient-friendly formulations of cefadroxil. For instance, the introduction of extended-release formulations allows for less frequent dosing, improving patient compliance. Market data indicates that such advancements are likely to enhance the therapeutic efficacy of cefadroxil, making it a preferred choice among healthcare providers. As a result, the Cefadroxil Market is expected to witness growth driven by these technological improvements, which not only optimize treatment outcomes but also expand the potential patient base.