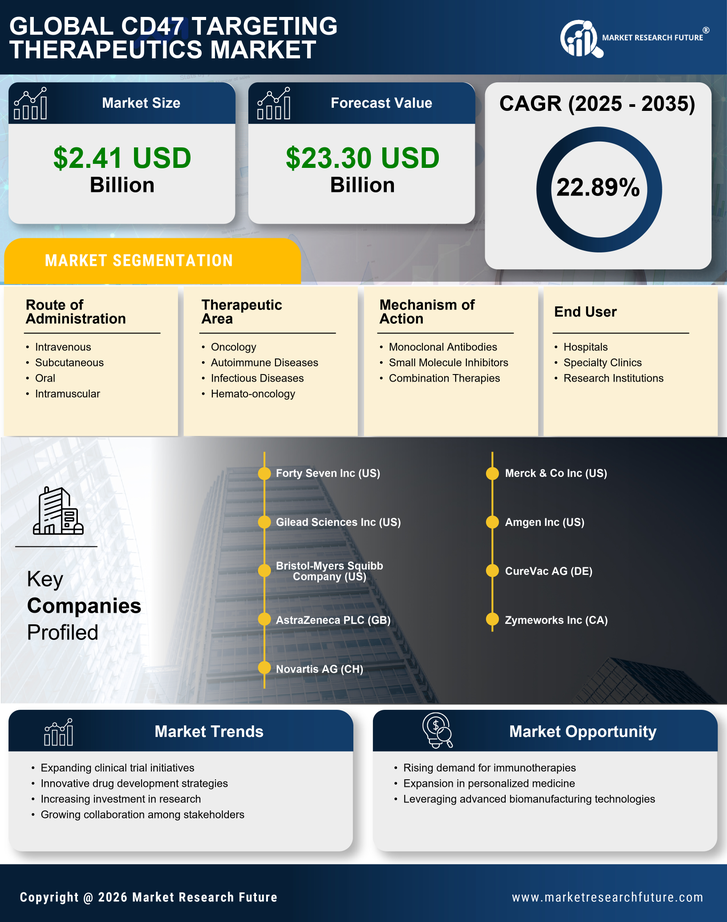
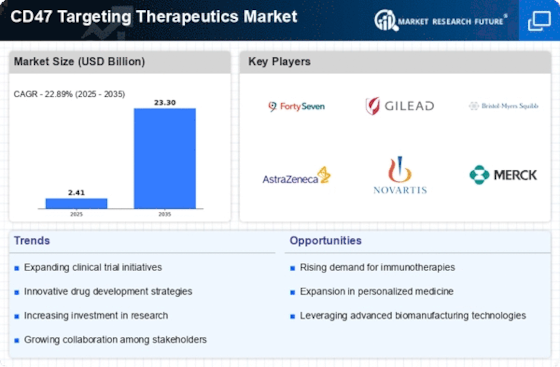
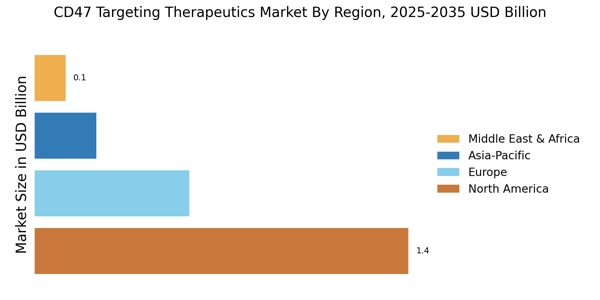
Increasing Cancer Prevalence
The rising incidence of cancer worldwide is a primary driver for the CD47 Targeting Therapeutics Market. As cancer remains a leading cause of mortality, the demand for innovative treatment options is escalating. According to recent statistics, cancer cases are projected to increase significantly, with estimates suggesting that by 2040, there could be nearly 30 million new cases annually. This alarming trend underscores the urgency for effective therapies, particularly those targeting immune evasion mechanisms like CD47. The CD47 Targeting Therapeutics Market is poised to benefit from this growing need, as therapies that inhibit CD47 can potentially enhance the immune system's ability to recognize and destroy cancer cells, thereby offering hope for improved patient outcomes.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is influencing the CD47 Targeting Therapeutics Market. As healthcare moves away from one-size-fits-all approaches, there is an increasing emphasis on tailoring treatments to individual patient profiles. CD47-targeting therapies are particularly well-suited for this trend, as they can be customized based on specific tumor characteristics and patient genetics. This personalized approach not only enhances treatment efficacy but also minimizes adverse effects, making it a preferred choice among oncologists. The market is witnessing a surge in demand for therapies that align with personalized medicine principles, indicating a robust growth trajectory for CD47-targeting agents.
Advancements in Research and Development
Ongoing advancements in research and development are propelling the CD47 Targeting Therapeutics Market forward. Innovative technologies, such as monoclonal antibodies and small molecule inhibitors, are being explored to enhance the efficacy of CD47-targeting therapies. Recent studies indicate that these therapies can significantly improve the immune response against tumors, leading to promising clinical trial results. The investment in R&D is substantial, with billions allocated annually to explore novel therapeutic approaches. This influx of funding and innovation is likely to accelerate the development of new CD47-targeting agents, thereby expanding the market and providing patients with more treatment options.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies, which is beneficial for the CD47 Targeting Therapeutics Market. Streamlined approval processes and incentives for breakthrough therapies are encouraging pharmaceutical companies to invest in CD47-targeting treatments. Recent regulatory frameworks have been established to expedite the development and approval of promising therapies, particularly those addressing unmet medical needs. This supportive environment is likely to foster innovation and accelerate the entry of new CD47-targeting agents into the market, ultimately enhancing treatment options for patients and driving market growth.
Collaborative Partnerships in Drug Development
Collaborative partnerships among pharmaceutical companies, academic institutions, and research organizations are emerging as a key driver for the CD47 Targeting Therapeutics Market. These collaborations facilitate the sharing of knowledge, resources, and expertise, which can significantly enhance the development of CD47-targeting therapies. By pooling resources, partners can conduct larger and more comprehensive clinical trials, thereby increasing the likelihood of successful outcomes. The trend towards collaboration is evident, with numerous partnerships being formed to advance research in this area. Such alliances are expected to accelerate the pace of innovation and bring new therapies to market more efficiently.