Advancements in Treatment Modalities
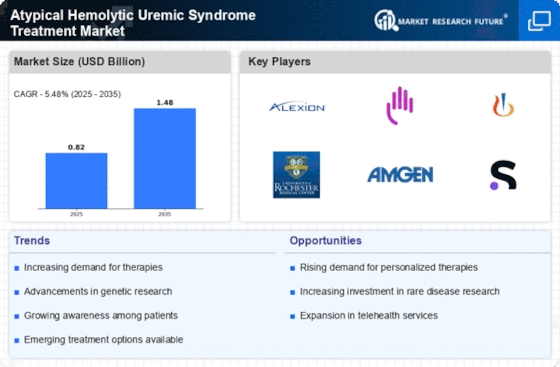
Innovations in treatment modalities are significantly influencing the Atypical Hemolytic Uremic Syndrome Treatment Market. The introduction of targeted therapies, such as complement inhibitors, has transformed the management of aHUS. These therapies have demonstrated efficacy in reducing the incidence of thrombotic microangiopathy and improving renal outcomes. Market data indicates that the global market for complement inhibitors is projected to reach several billion dollars by the end of the decade, reflecting the growing investment in research and development. Additionally, the emergence of personalized medicine approaches is likely to enhance treatment efficacy, further driving market growth. As new therapies continue to be developed and approved, the treatment landscape for aHUS is expected to evolve, offering patients more options and potentially better outcomes.
Increased Research Funding and Investment
The surge in research funding and investment in rare diseases, particularly atypical hemolytic uremic syndrome, is a crucial driver for the Atypical Hemolytic Uremic Syndrome Treatment Market. Governments and private organizations are increasingly recognizing the need for research into rare conditions, leading to enhanced funding opportunities. This influx of capital is likely to accelerate the development of novel therapies and improve existing treatment protocols. Market analysts suggest that the total funding for rare disease research has seen a significant uptick, with millions allocated specifically for aHUS studies. This financial support not only fosters innovation but also encourages collaboration among pharmaceutical companies, academic institutions, and healthcare providers, ultimately benefiting patients through improved treatment options.
Growing Patient Advocacy and Support Groups
The rise of patient advocacy and support groups is playing a pivotal role in shaping the Atypical Hemolytic Uremic Syndrome Treatment Market. These organizations are instrumental in raising awareness about aHUS, educating patients and healthcare professionals about the condition, and advocating for better treatment options. Increased visibility of aHUS through these groups has led to heightened demand for effective therapies. Furthermore, patient advocacy groups often collaborate with pharmaceutical companies to facilitate clinical trials and research initiatives, thereby accelerating the development of new treatments. As these organizations continue to grow in influence, they are likely to drive market expansion by fostering a more informed patient population and promoting the need for innovative therapies.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant factor influencing the Atypical Hemolytic Uremic Syndrome Treatment Market. Regulatory agencies are increasingly prioritizing the approval of novel treatments for rare diseases, including aHUS. This trend is evident in the expedited review processes and incentives provided for companies developing therapies for conditions with unmet medical needs. The approval of new treatments not only enhances patient access to effective therapies but also stimulates market growth. Recent data indicates that the time to market for new aHUS therapies has decreased due to favorable regulatory environments, which may encourage further investment in research and development. As regulatory frameworks continue to evolve, they are likely to facilitate the introduction of innovative therapies, thereby expanding the treatment landscape for aHUS.
Rising Incidence of Atypical Hemolytic Uremic Syndrome
The increasing incidence of atypical hemolytic uremic syndrome (aHUS) is a primary driver for the Atypical Hemolytic Uremic Syndrome Treatment Market. Recent estimates suggest that the prevalence of aHUS is approximately 2 to 3 cases per million people annually. This rising incidence necessitates the development and availability of effective treatment options, thereby propelling market growth. As healthcare providers become more aware of the condition, the demand for specialized therapies is likely to increase. Furthermore, the growing recognition of aHUS as a serious condition has led to enhanced diagnostic capabilities, which may contribute to earlier detection and treatment. Consequently, the market is expected to expand as more patients are diagnosed and treated for this rare but severe disorder.