Increase in Asthma Prevalence
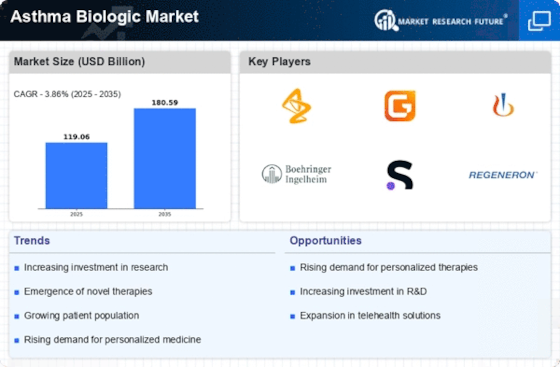
The rising prevalence of asthma worldwide is a pivotal driver for the Asthma Biologic Market. Recent estimates indicate that asthma affects approximately 300 million individuals globally, with this number projected to increase. This growing patient population necessitates innovative treatment options, particularly biologics, which target specific pathways involved in asthma pathophysiology. As healthcare providers seek effective therapies to manage asthma symptoms and reduce exacerbations, the demand for biologics is likely to surge. Furthermore, the increasing awareness of asthma management and the importance of personalized treatment plans contribute to the expansion of the Asthma Biologic Market. Consequently, pharmaceutical companies are investing in research and development to create novel biologic therapies that cater to the unique needs of asthma patients.
Regulatory Support for Biologics
Regulatory support for biologics is an essential driver for the Asthma Biologic Market. Regulatory agencies are increasingly recognizing the importance of biologic therapies in treating chronic conditions like asthma. Streamlined approval processes and expedited pathways for biologics are encouraging pharmaceutical companies to bring new therapies to market more efficiently. For example, the introduction of breakthrough therapy designations has facilitated faster access to innovative treatments for asthma patients. This supportive regulatory environment not only enhances the attractiveness of investing in biologic development but also fosters competition within the Asthma Biologic Market. As more biologics gain approval, the market is likely to expand, providing patients with a wider array of treatment options.
Advancements in Biologic Therapies
The continuous advancements in biologic therapies represent a significant driver for the Asthma Biologic Market. Recent innovations have led to the development of monoclonal antibodies and other biologics that specifically target inflammatory pathways associated with asthma. For instance, therapies such as omalizumab and mepolizumab have demonstrated efficacy in reducing asthma exacerbations and improving overall patient outcomes. The market for these biologics is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of over 10% in the coming years. As more biologics receive regulatory approval and enter the market, healthcare providers are increasingly adopting these therapies, thereby enhancing the Asthma Biologic Market. This trend indicates a shift towards more targeted and effective treatment options for asthma patients.
Growing Investment in Research and Development
The growing investment in research and development (R&D) within the pharmaceutical sector is a crucial driver for the Asthma Biologic Market. Companies are allocating substantial resources to discover and develop new biologic therapies that address unmet medical needs in asthma management. This trend is evidenced by the increasing number of clinical trials focused on biologics, with hundreds currently underway. The investment in R&D not only fosters innovation but also enhances the competitive landscape of the Asthma Biologic Market. As new therapies emerge, they are likely to provide improved efficacy and safety profiles, attracting more patients and healthcare providers. This influx of novel treatments may also lead to a broader acceptance of biologics as a standard of care for asthma.
Rising Awareness and Education on Asthma Management
The rising awareness and education surrounding asthma management are significant drivers for the Asthma Biologic Market. Increased public health campaigns and educational initiatives have led to a better understanding of asthma and its treatment options among patients and healthcare providers. This heightened awareness encourages patients to seek effective therapies, including biologics, which are often viewed as advanced treatment options. As patients become more informed about the benefits of biologics, the demand for these therapies is expected to rise. Additionally, healthcare professionals are increasingly recognizing the importance of personalized treatment approaches, further driving the adoption of biologics in the Asthma Biologic Market. This trend suggests a shift towards more proactive asthma management strategies, ultimately benefiting patient outcomes.