-
Report Prologue
-
Market Introduction
-
Introduction
-
Scope of Study
- Research Objective
- Assumptions
- Limitations
-
Research Methodology
-
Introduction
-
Primary Research
-
Secondary research
-
Market
-
Size Estimation
-
Market Dynamics
-
Drivers
-
Restrains
-
Opportunities
-
Challenges
-
Macroeconomic Indicators
-
Technology Trends & Assessment
-
Market Factor Analysis
-
Porters
- Bargaining Power of Suppliers
- Bargaining
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Five Forces Analysis
-
Power of Buyers
-
Value Chain Analysis
-
Investment
-
Feasibility Analysis
-
Pricing Analysis
-
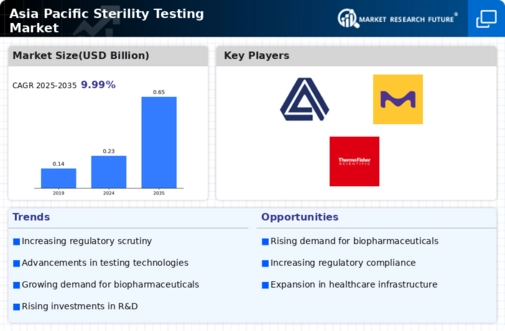
Asia Pacific Sterility Testing
-
Asia Pacific Sterility Testing Market, by Type
-
Introduction
-
Instrument
- Market Estimates
-
& Forecast, 2020-2027
-
Reagent & Services
- Market Estimates
-
& Forecast, 2020-2027
-
Kits
- Market Estimates & Forecast,
-
Others
-
Asia Pacific Sterility Testing Market, by Type
-
of Tests
-
Introduction
-
Membrane Filtration Sterility Testing
- Market Estimates & Forecast, 2020-2027
-
Direct Incubation Sterility
- Market Estimates & Forecast, 2020-2027
-
Testing
-
Direct Transfer
- Market Estimates & Forecast, 2020-2027
-
Others
-
Asia
-
Asia Pacific Sterility Testing Market, by Application
-
Introduction
- Market Estimates &
-
Pharmaceutical & Biological Manufacturing
-
Forecast, 2020-2027
-
Medical Devices Manufacturing
- Market Estimates
-
& Forecast, 2020-2027
-
Others
-
Asia Pacific Sterility Testing
-
Asia Pacific Sterility Testing Market, by End user
-
Introduction
-
Pharmaceuticals
- Market
-
Estimates & Forecast, 2020-2027
-
Hospital & Laboratories
-
Market Estimates & Forecast, 2020-2027
-
Others
-
Asia Pacific
-
Asia Pacific Sterility Testing Market, by Region
-
Introduction
-
Asia Pacific
- Japan
- China
- India
- Republic of Korea
- Australia
- Rest of Asia Pacific
-
Competitive Landscape
-
Introduction
-
Market Share Analysis
-
Key Development
- Key Developments
-
& Strategies
-
Company Profiles
-
Boston Scientific Corporation
-
Overview
-
Laboratories International, Inc.
-
Overview
-
SWOT Analysis
-
Product Overview
-
Overview
-
Scientific, Inc.
-
Overview
-
SWOT Analysis
-
Key Development
-
Company Overview
-
Overview
-
KGaA
-
Company Overview
-
Product
-
Financials
-
SWOT Analysis
-
Charles River
- Company Overview
- Product
- Financial Overview
- Key Developments
-
Avance Biosciences
- Company Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Paragon Bioservices, Inc
- Company
- Product/Business Segment Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Thermo Fisher
- Company Overview
- Product/Business Segment
- Financial Overview
- Key Development
-
Avista Pharma Solutions
- Company Overview
- Product/Business Segment Overview
- Financial Overview
- SWOT Analysis
-
DYNALABS LLC.
- Product/Business Segment Overview
- Financial
- Key Development
- SWOT Analysis
-
Merck
- Company Overview
- Product/Business Segment Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Others
-
MRFR Conclusion
-
Key Findings
- From
- Unmet Needs of the Market
-
CEO’s View Point
-
Key Companies
-
to Watch
-
Prediction of Pharmaceutical Industry
-
Appendix
-
LIST
-
OF TABLES
-
Sterility testing Industry Synopsis, 2020-2027
-
Table
-
Asia Pacific Sterility Testing Market Estimates and Forecast, 2020-2027, (USD
-
Million)
-
Asia Pacific Sterility Testing Market by Region, 2020-2027,
-
(USD Million)
-
Asia Pacific Sterility Testing Market by Types, 2020-2027,
-
(USD Million)
-
Asia Pacific Sterility Testing Market by Tests, 2020-2027,
-
(USD Million)
-
Asia Pacific Sterility Testing Market by Application,
-
Asia Pacific Sterility Testing Market by End
-
Users, 2020-2027, (USD Million)
-
Japan Sterility Testing Market by Types,
-
Japan Sterility Testing Market by Tests, 2020-2027,
-
(USD Million)
-
Japan Sterility Testing Market by Application, 2020-2027,
-
(USD Million)
-
Japan Sterility Testing Market by End Users, 2020-2027,
-
(USD Million)
-
China Sterility Testing Market by Types, 2020-2027,
-
(USD Million)
-
China Sterility Testing Market by Tests, 2020-2027,
-
(USD Million)
-
China Sterility Testing Market by Application, 2020-2027,
-
(USD Million)
-
China Sterility Testing Market by End Users, 2020-2027,
-
(USD Million)
-
India Sterility Testing Market by Types, 2020-2027,
-
(USD Million)
-
India Sterility Testing Market by Tests, 2020-2027,
-
(USD Million)
-
India Sterility Testing Market by Application, 2020-2027,
-
(USD Million)
-
India Sterility Testing Market by End Users, 2020-2027,
-
(USD Million)
-
Republic of Korea Sterility Testing Market by Types,
-
Republic of Korea Sterility Testing Market
-
by Tests, 2020-2027, (USD Million)
-
Republic of Korea Sterility Testing
-
Market by Application, 2020-2027, (USD Million)
-
Republic of Korea
-
Sterility Testing Market by End Users, 2020-2027, (USD Million)
-
Rest
-
of Asia Pacific Sterility Testing Market by Types, 2020-2027, (USD Million)
-
Table
-
Rest of Asia Pacific Sterility Testing Market by Tests, 2020-2027, (USD Million)
-
Rest of Asia Pacific Sterility Testing Market by Application, 2020-2027,
-
(USD Million)
-
Rest of Asia Pacific Sterility Testing Market by End
-
Users, 2020-2027, (USD Million)
-
LIST OF FIGURES
-
Research
-
Process
-
Segmentation for Asia Pacific Sterility Testing Market
-
Segmentation Market Dynamics for Sterility Testing Market
-
Figure
-
Asia Pacific Sterility Testing Market, by Types 2020
-
Asia
-
Asia Pacific Sterility Testing Market, by Tests 2020
-
Asia Pacific
-
Asia Pacific Sterility Testing Market, by Applications, 2020
-
Asia Pacific
-
Asia Pacific Sterility Testing Market, by End Users, 2020
-
Asia Pacific Sterility
-
Asia Pacific Sterility Testing Market, by Region, 2020
-
Asia Pacific Sterility Testing
-
Market: Company Share Analysis, 2020 (%)
-
Boston Scientific Corporation:
-
Key Financials
-
Boston Scientific Corporation: Segmental Revenue
-
Boston Scientific Corporation: Geographical Revenue
-
Charles
-
River Laboratories International, Inc.: Key Financials
-
Charles River
-
Laboratories International, Inc.: Segmental Revenue
-
Charles River
-
Laboratories International, Inc.: Geographical Revenue
-
Avance Biosciences:
-
Key Financials
-
Avance Biosciences: Segmental Revenue
-
Figure
-
Avance Biosciences: Geographical Revenue
-
Paragon Bioservices,
-
Inc: Key Financials
-
Paragon Bioservices, Inc: Segmental Revenue
-
Paragon Bioservices, Inc: Geographical Revenue
-
Thermo
-
Fisher Scientific, Inc.: Key Financials
-
Thermo Fisher Scientific,
-
Inc.: Segmental Revenue
-
Thermo Fisher Scientific, Inc.: Geographical
-
Revenue
-
Avista Pharma Solutions: Key Financials
-
Avista
-
Pharma Solutions: Segmental Revenue
-
Avista Pharma Solutions: Geographical
-
Revenue
-
Dynalabs LLC: Key Financials
-
Dynalabs LLC:
-
Segmental Revenue
-
Dynalabs LLC: Geographical Revenue
-
Figure
-
Merck KGaA: Key Financials
-
Merck KGaA: Segmental Revenue
-
Figure
-
Merck KGaA: Geographical Revenue













Leave a Comment