Technological Advancements
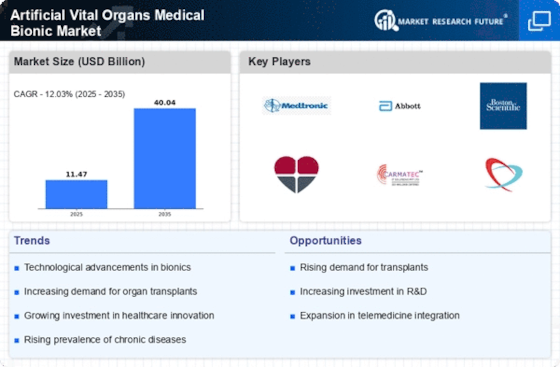
The Artificial Vital Organs Medical Bionic Market is experiencing rapid technological advancements that enhance the functionality and efficiency of bionic organs. Innovations in materials science, such as biocompatible polymers and advanced alloys, are enabling the development of more durable and effective artificial organs. Furthermore, the integration of artificial intelligence and machine learning into these devices allows for real-time monitoring and adaptive responses to physiological changes. According to recent data, the market for artificial organs is projected to grow at a compound annual growth rate of approximately 15% over the next five years, driven by these technological improvements. This growth indicates a strong demand for innovative solutions that can improve patient outcomes and quality of life.
Rising Healthcare Expenditure
The increasing healthcare expenditure across various regions is propelling the growth of the Artificial Vital Organs Medical Bionic Market. As healthcare systems allocate more resources towards advanced medical technologies, the demand for artificial organs is expected to rise correspondingly. Data suggests that healthcare spending is projected to reach over 10 trillion dollars by 2027, with a significant portion directed towards innovative treatments and technologies. This trend indicates a favorable environment for the development and adoption of artificial vital organs, as healthcare providers seek to improve patient care and outcomes through advanced medical solutions.
Regulatory Support and Funding
Regulatory bodies are increasingly recognizing the importance of artificial vital organs, leading to enhanced support and funding for research and development within the Artificial Vital Organs Medical Bionic Market. Governments are providing grants and incentives to encourage innovation in this field, which is crucial for accelerating the approval processes for new devices. This regulatory environment fosters collaboration between public and private sectors, facilitating the development of cutting-edge technologies. As a result, the market is likely to see a rise in the number of approved products, which could potentially double in the next five years, thereby expanding access to life-saving bionic solutions.
Growing Awareness and Acceptance
There is a notable increase in public awareness and acceptance of artificial vital organs, which is positively influencing the Artificial Vital Organs Medical Bionic Market. Educational campaigns and success stories of patients benefiting from bionic organs are helping to demystify these technologies. As more individuals become informed about the potential benefits and advancements in artificial organs, the stigma surrounding their use diminishes. This shift in perception is likely to lead to higher demand for bionic solutions, with market analysts predicting a potential increase in adoption rates by 20% over the next few years. Such trends indicate a promising future for the integration of artificial vital organs into mainstream healthcare.
Increasing Prevalence of Organ Failure
The rising incidence of organ failure due to chronic diseases such as diabetes, hypertension, and liver disease is a significant driver for the Artificial Vital Organs Medical Bionic Market. As the global population ages, the demand for artificial organs is expected to surge. Reports indicate that organ failure affects millions of individuals, leading to a growing need for life-saving interventions. The market is responding to this urgent need, with an estimated increase in the number of artificial organ implants projected to reach over 500,000 annually by 2030. This trend underscores the critical role that artificial vital organs play in addressing the healthcare challenges posed by organ failure.