Market Trends
Key Emerging Trends in the Aortic Valve Market
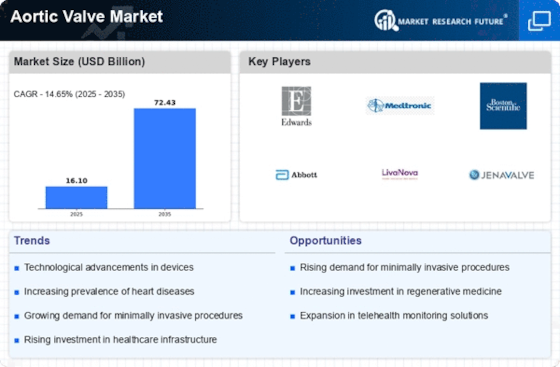
The Aortic Valve Market sector is on a revolutionary wave developing in the context of improved medical technology, widening population aging, and rising rates of cardiovascular diseases. Notable becoming is the hiking of transcatheter aortic valve replacement (TAVR) through the use of less an invasive method of replacing surgical valve. TAVR procedures have today became widespread use as consecutively they are less invasive, recovery time is significantly reduced, and broad applicability to patients with high risk for surgery and even those considered unsuitable. This indicates movement towards the new way of approaching aortic valve interventions, which require the least invasive methods for achieving better results in patients.
The med-tech market is experiencing the perfect time for the development of TAVR devices and systems. New technological developments in TAVR - such as valve designs, delivery systems and imaging procedures - result in the centralization and widespread use of TAVR procedures. However, this recent development is in line with long-term goals of improving the performance, efficacy and durability of TAVR thus widening indications of TAVR procedure to more patients.
In addition there is an increasing interest in the growing field of next-generation tissue and mechanical valves alongside the surgical and transcatheter interventions. The evolution of biomaterials, durability, and better in terms of the hemodynamic performance of prosthetic aortic valves continues to be the goal in research and development so as to extend their durability and functionality. Hence, it signals attempts to minimize the shortcomings usually experienced with valve replacement with a view to achieving long-term endurance and effectiveness.
The market now has to go through a trend that will place patient specific and precision medicine as the basis of aortic valve procedures. Through incorporating the most recent imaging, third dimension (3D) printing and artificial intelligence; patient specific treatment planning and device selection gets simplified and the desired winning result come to fruition. This tendency is the reflection of process-driven and individualized treatments of the aortic valve replacement, and of course this makes sense in context of precision medicine.
Technological inventions in percutaneous and catheter-based aortic valve surgeries determine the aortic valve market size and outlook. The utilization of improved tools, methods, and methods of deployment increases the procedural efficiency and safety of aortic valve operation as well. The increasing trend is an indicator of the ongoing movement in the interventional cardiology field that leads to more precise and controlled valve implantations.
In addition, the current segment is dilated with a growth in the utilization of aortic valve restoration techniques. The valve replacement surgery still continues to be an ace of practices; however, there is an increasing attention to repair procedures that retain the patients’ native valves. The shift towards these techniques matches the period of development where there is a quest for treatment procedures that offer the least harm but at the same time, they are life saving in nature.

















Leave a Comment