Increase in Incidence Rates
The rising incidence rates of anaplastic astrocytoma are a primary driver for the Anaplastic Astrocytoma Market. Recent data indicates that the annual incidence of gliomas, including anaplastic astrocytoma, has been increasing, with estimates suggesting approximately 3.2 cases per 100,000 individuals. This trend is likely attributed to various factors, including environmental influences and improved diagnostic capabilities. As more cases are identified, the demand for effective treatment options and innovative therapies is expected to grow, thereby propelling the market forward. Furthermore, the increasing awareness among healthcare professionals and patients regarding brain tumors contributes to early diagnosis and intervention, which may enhance survival rates and quality of life for patients. Consequently, this growing incidence is anticipated to stimulate investment in research and development within the Anaplastic Astrocytoma Market.
Increased Awareness and Education
The heightened awareness and education surrounding anaplastic astrocytoma are pivotal in shaping the Anaplastic Astrocytoma Market. Initiatives aimed at educating healthcare professionals and the public about brain tumors have gained momentum, leading to earlier diagnosis and treatment. Organizations dedicated to brain cancer advocacy are actively promoting awareness campaigns, which have been shown to improve patient outcomes. As awareness increases, more individuals are likely to seek medical attention for symptoms associated with anaplastic astrocytoma, resulting in higher diagnosis rates. This trend is further supported by the proliferation of online resources and support networks that provide valuable information to patients and caregivers. Consequently, the growing awareness is expected to drive demand for diagnostic and therapeutic options within the Anaplastic Astrocytoma Market.
Advancements in Treatment Modalities
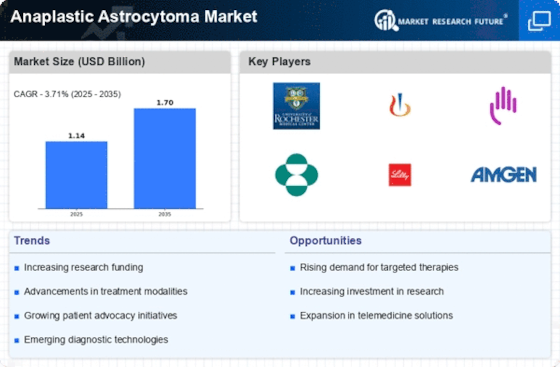
Innovations in treatment modalities are significantly influencing the Anaplastic Astrocytoma Market. The development of targeted therapies and immunotherapies has shown promise in improving patient outcomes. For instance, the introduction of novel chemotherapeutic agents and combination therapies has been associated with enhanced efficacy in managing anaplastic astrocytoma. Market data suggests that the global market for brain cancer therapeutics is projected to reach USD 3.5 billion by 2026, reflecting a compound annual growth rate of approximately 7.5%. These advancements not only provide new hope for patients but also attract substantial investments from pharmaceutical companies aiming to develop cutting-edge treatments. As a result, the ongoing research and clinical trials focusing on innovative therapies are likely to drive growth in the Anaplastic Astrocytoma Market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a significant driver in the Anaplastic Astrocytoma Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for breakthrough therapies that demonstrate potential in treating aggressive brain tumors. Initiatives such as the FDA's Breakthrough Therapy Designation aim to facilitate the development and review of promising treatments, thereby accelerating their availability to patients. This supportive regulatory environment encourages pharmaceutical companies to invest in the development of novel therapies for anaplastic astrocytoma. As a result, the market is likely to witness a surge in the introduction of innovative treatment options, which could enhance patient outcomes and drive market growth. The alignment of regulatory frameworks with scientific advancements is expected to foster a conducive environment for the Anaplastic Astrocytoma Market.
Growing Investment in Research and Development
The increasing investment in research and development (R&D) is a crucial driver for the Anaplastic Astrocytoma Market. Pharmaceutical companies and research institutions are allocating significant resources to explore novel therapeutic approaches and improve existing treatment protocols. This trend is underscored by the fact that R&D spending in the oncology sector has been on the rise, with estimates indicating that it could exceed USD 200 billion by 2025. Such investments are essential for advancing the understanding of anaplastic astrocytoma biology and identifying potential biomarkers for targeted therapies. Moreover, collaborations between academia and industry are fostering innovation, leading to the development of new drugs and treatment strategies. As R&D efforts continue to expand, they are expected to enhance the therapeutic landscape for anaplastic astrocytoma, thereby propelling the market forward.