Focus on Personalized Medicine
The emphasis on personalized medicine is reshaping the Active Pharmaceutical Ingredients Market. As healthcare evolves towards more tailored treatment approaches, the demand for specific active pharmaceutical ingredients that cater to individual patient needs is increasing. This trend is driven by advancements in genomics and biotechnology, which enable the development of targeted therapies. Pharmaceutical companies are investing in the research and production of active pharmaceutical ingredients that align with personalized treatment regimens. The market for personalized medicine is expected to grow significantly, with estimates suggesting it could reach USD 2 trillion by 2030. This growth presents substantial opportunities for active pharmaceutical ingredients, as they are integral to the formulation of personalized therapies.
Rising Demand for Generic Drugs
The growing acceptance and demand for generic drugs are driving the Active Pharmaceutical Ingredients Market. As healthcare costs continue to rise, patients and healthcare providers are increasingly turning to generic alternatives, which are often more affordable than their branded counterparts. This shift is prompting pharmaceutical companies to invest in the production of active pharmaceutical ingredients for generics, thereby expanding their market presence. Data suggests that the generic drug market is projected to reach a value of over USD 500 billion by 2026, indicating a robust growth trajectory. Consequently, the active pharmaceutical ingredients market is likely to benefit from this trend, as the production of generics requires a steady supply of high-quality active pharmaceutical ingredients.
Regulatory Support and Initiatives
Regulatory bodies play a crucial role in shaping the Active Pharmaceutical Ingredients Market through supportive policies and initiatives. Governments are increasingly recognizing the importance of ensuring the availability of high-quality active pharmaceutical ingredients to safeguard public health. Initiatives aimed at streamlining the approval process for new drugs and active pharmaceutical ingredients are being implemented, which may enhance market accessibility. Furthermore, regulatory frameworks that promote transparency and compliance are likely to encourage investment in the active pharmaceutical ingredients sector. This supportive environment is expected to facilitate the entry of new players into the market, thereby fostering competition and innovation.
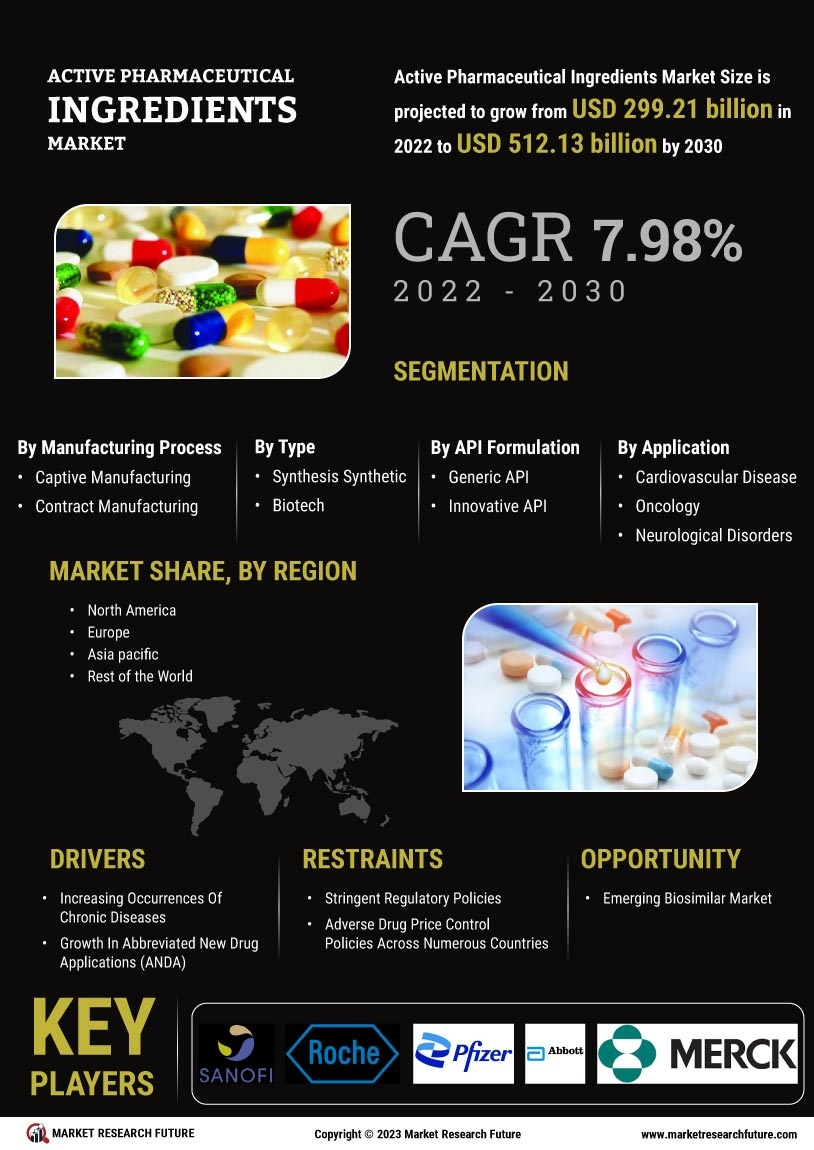
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as diabetes, cancer, and cardiovascular disorders is a primary driver of the Active Pharmaceutical Ingredients Market. As healthcare systems strive to address these conditions, the demand for effective pharmaceuticals increases, leading to a heightened need for active pharmaceutical ingredients. According to recent data, chronic diseases account for approximately 70% of all deaths worldwide, underscoring the urgency for innovative treatments. This trend compels pharmaceutical companies to invest in research and development, thereby expanding the active pharmaceutical ingredients market. The growing patient population necessitates a robust supply chain for active pharmaceutical ingredients, which is likely to stimulate market growth in the coming years.
Advancements in Pharmaceutical Research and Development
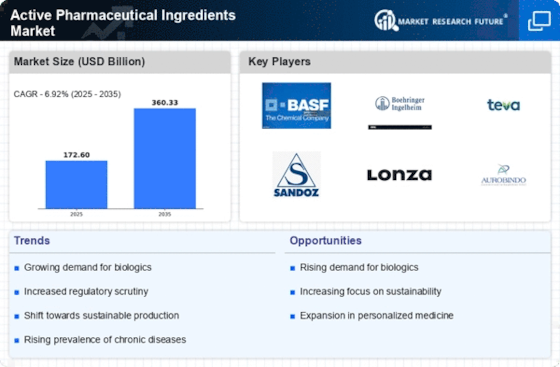
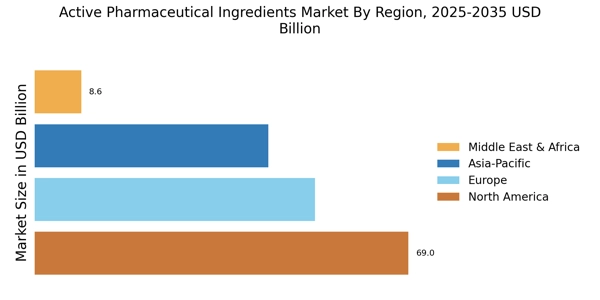
Technological advancements in pharmaceutical research and development are significantly influencing the Active Pharmaceutical Ingredients Market. Innovations in drug formulation, delivery systems, and manufacturing processes enhance the efficiency and effectiveness of active pharmaceutical ingredients. For instance, the integration of artificial intelligence and machine learning in drug discovery accelerates the identification of potential compounds, thereby reducing time-to-market for new drugs. This dynamic environment fosters competition among pharmaceutical companies, driving them to seek high-quality active pharmaceutical ingredients. As a result, the market is expected to witness substantial growth, with projections indicating a compound annual growth rate of over 6% in the next five years.