QIAGEN & Helix Partners to Develop Companion Diagnostics Products for Hereditary Diseases
The demand for companion diagnostics devices and tests that identify clinically relevant genetic abnormalities increases as the development of precision medicines quickens. By identifying patients most likely to benefit from a particular medicinal product or to be at heightened risk, these diagnostics aid clinical decision-making. Companion diagnostics that use whole exome sequencing are mostly used in oncology, but they are widely thought to have significant potential in hereditary disease areas like cardiovascular, metabolic, neuro-degenerative, and auto-immune disorders.
On January 5th, 2023, QIAGEN announced it had entered a strategic partnership with Helix, a US-based population genetics market leader to work together and advance companion diagnostics efforts aimed at hereditary diseases. Through this partnership, QIAGEN will become the exclusive contracting and marketing partner for Helix’s companion diagnostics services. Additionally, QIAGEN will be able to leverage Helix’s laboratory platforms which had received the first-ever US-FDA Class II de novo authorization for whole genome sequencing. Helix will also benefit from getting access to QIAGEN’s diagnostics expertise and its global reach.
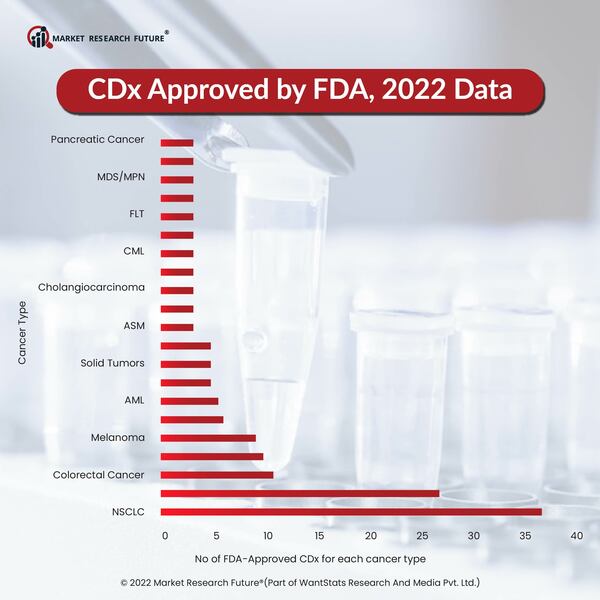
QIAGEN has over 30 major collaborations with key pharmaceutical and biotechnology companies globally to develop diagnostic solutions for their drug products. The companion diagnostic products offered by QIAGEN cover a range of technologies, including next-generation sequencing (NGS), polymerase chain reaction (PCR), and digital PCR (dPCR), sample types, including biopsy, and disease areas, including cancer and Parkinson's. There are 11 PCR-based companion diagnostics that have received FDA approval, and a collaboration with Neuron23 to develop an NGS-based companion diagnostic for a novel Parkinson's disease drug was announced in September 2022.
Health systems, life sciences firms, and payers can enhance genomic research and hasten the integration of genomic data into clinical care thanks to end-to-end platform that Helix has created from in order to allow population genomics initiatives with at least 100,000 patients each across the U.S., Helix has teamed with top health systems. These programmes power real-world data (RWD) or real-world evidence (RWE) services and insights and bring deep genetic expertise and methodologies to examine sub-cohorts within both drug discovery and a clinical trial. These programmes also aid in the patient identification and recruitment for clinical trials in hereditary diseases like Parkinson's, cardiovascular or inflammatory diseases like non-alcoholic steatohepatitis (NASH), and power real-world data (RWD) or real-world evidence (RWE) services and insights.
Additionally, according to Thierry Bernard, Chief Executive Officer of QIAGEN, this partnership will also lead to significant developments in the clinical trial space. It will give access to a genomic database that will aid researchers to identify a patient with specific genomic biomarkers in very short intervals, which will shorten trail recruitment steps to a few months from years.