Everything You Should Know About Ester
Understanding Esters: Structure, Reactions, and Industrial Applications
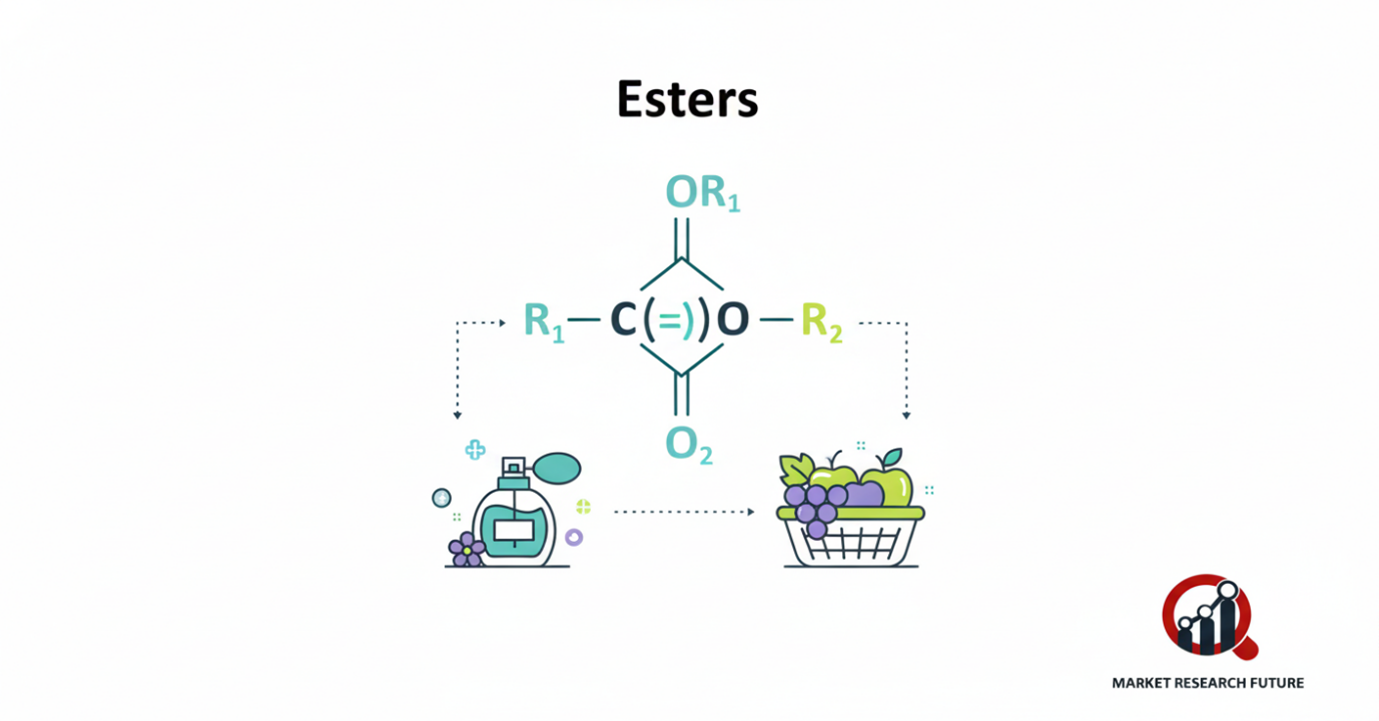
Esters are interesting organic compounds for many reasons. They produce the delicious smells of ripe fruit, the softening of materials, and the manufacture of highly explosive materials. In organic chemistry, an ester is produced when an alcohol and an acid (usually a carboxylic acid or phenol) react to form water. This transformation is called esterification. The compounds produced are essential to the food industry, cosmetics, and manufacturing.
Esters are common in fruits, fats, and oils, which provide their distinctive taste and smell. In the synthetic world, they are used to make perfumes, food additives, and industrial solvents. The explosive nitroglycerin, a glycerol ester and nitric acid, can also treat fatal heart conditions. DNA contains phosphoric acid esters, and others have been used in the past as insecticides or chemical warfare. The sulfuric acid esters range from the poisonous dimethyl sulfate to long-chain alcohol sulfates that are used in detergents.
Origin and Naming of Esters
Esters are responsible for the smell of a variety of fruits including strawberries, apples, and pineapples. In 1850, the German chemist Leopold Gmelin, referred to the compound as an essigäther or "vinegar ether." Most esters are named based on the parent carboxylic acid, and typically, the name will end with the suffix "-ate." For example, acetic acid (ethyl acetate) can also be classified as an acetic acid ethyl ester.
The Process Involving Esters
Esterification occurs as an equilibrium reaction in which the forward and backward reactions proceed slowly. Chemists subdue this condition using strong acids like concentrated sulfuric acid which catalyze the reaction. In this case, a proton is transferred to the acid's carbonyl group and the bond with the carbon is broken. Rearrangements which generate water and the ester occurs.
The reverse reactions, in which the ester bond is broken using water and acids, enzymes, or alkali, is called hydrolysis or saponification. In fats, for example, glycerol and fatty acids form triglycerides. When these triglycerides are heated with sodium hydroxide, the reaction yields glycerol and sodium stearate (soap) which is a major component of soap. This process made a huge impact on cleaning products.
Example of a Reactions: Triglycerides + 3 NaOH = Glycerine + Sodium stearate (soap)
Chemical Behavior of Esters
- Acid Hydrolysis: If an ester is heated with a large quantity of water and replaced with the water vapor, the ester is decomposed to the carboxylic acid and the corresponding alcohol, which is the reverse of the esterification process.
- Alkaline Hydrolysis: The decomposition of an ester with an alkali is a fundamental reaction. NaOH with the ester will produce the corresponding alcohol and the sodium salt of the carboxylic acid. This reaction is the basis for the manufacture of soaps and biodiesels.
Common Uses of Esters
Esters are used in manufacture of a vast array of everyday items: The construction and clothing industries use and Polymerized Esters to create Plexiglas (transparent acrylic) and Dacron (polyester fiber).
Fats as well as oils are natural esters formed from glycerol and fatty acid. Fats are solids while oils are liquids. Both are crucial to nutrition and biochemistry.
Waxes are long chained carboxylic oils along with alcohols, and they also have a natural protective function. An example is beeswax.
Some small chain esters are volatile and have a strong fruity pleasant smell. This is why they are used in the manufacture of perfumes, candies, and soft beverages.
Conclusion
Esters have low solubility in water and low density. They should be handled with care because they can irritate the airways and have minor narcotic effects. Besides being used in flavorings, esters are also used as industrial solvents, plasticizers, and as intermediates in the synthesis of other compounds, which shows the practical side of chemistry in our daily lives. Esters are the perfect balance of chemistry and art. They are the invisible compounds that shape our world, from the smell of ripe fruits to the strength of materials we use every day.

Leave a Comment