Ethanol: its Utilities in Medical Industry and as a Fuel
The Introduction to the Ethanol Market
Ethanol finds vast applications as fuel in various end-use segments. This escalates the scope of ethanol, upholding the size of the global ethanol market to USD 98.44 billion in 2024. Projections predict that the valuation of the sector will reach USD 201.36 billion by 2035, owing to a moderate CAGR of 6.72% through the forecast period.
Ethanol, also known as drinking alcohol, spirit, or ethyl alcohol, is an organic chemical compound with the formula C₂H₆O. It is a flammable, colorless, volatile liquid with a distinctive smell similar to methanol.
Ethanol can be naturally produced from sugars through fermentation or synthetically via petrochemical processes such as ethylene hydration. Its versatile properties make it valuable in the medical industry, as a fuel, and as a chemical solvent.
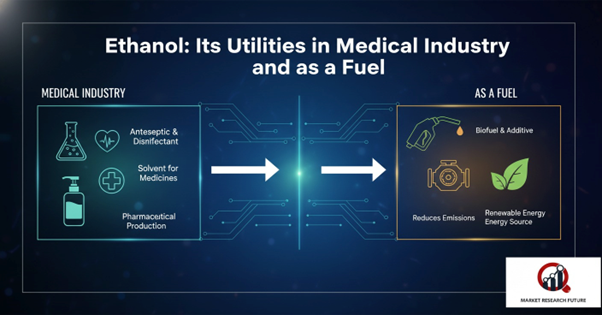
Various Uses of Ethanol
- Antiseptic: Ethanol is widely used in medical wipes and hand sanitizers due to its bactericidal, antifungal, and antiviral properties. It effectively kills microorganisms and helps prevent infections.
- Antidote: It can be administered to treat methanol and ethylene glycol poisoning.
- Medicinal Solvent: Ethanol dissolves medications and other compounds insoluble in water, acting as an antimicrobial preservative in liquid pharmaceutical preparations.
- Pharmacology: Orally consumed ethanol is metabolized efficiently by the liver.
- Recreational Purposes: As a central nervous system depressant, ethanol is widely consumed for its psychoactive effects.
- Fuel: Ethanol serves as engine fuel and additive, and is used in rocket fuel and lightweight racing aircraft.
- Household Heating: Ethanol fireplaces are popular for both decoration and home heating.
- Feedstock: It is a precursor for compounds like ethyl esters, acetic acid, and ethyl amines.
- Solvent: Ethanol dissolves both polar and non-polar compounds, making it essential in paints, markers, perfumes, and mouthwashes.
- Low-Temperature Liquid: In laboratories, ethanol serves as a cooling bath with dry ice to maintain sub-zero temperatures.
Regional Analysis
The demand for ethanol varies regionally, driven by industrial, medical, and fuel applications. North America leads in ethanol production and consumption due to its extensive pharmaceutical industry, high demand for biofuels, and strict hygiene standards.
Europe closely follows, with Germany, France, and the UK investing heavily in bioethanol as a renewable fuel and in healthcare products.
The Asia-Pacific region, particularly India and China, is experiencing rapid growth due to expanding pharmaceutical manufacturing, increased adoption of hand sanitizers, and government incentives for biofuel production.
Latin America benefits from abundant sugarcane resources, making Brazil a global leader in ethanol fuel production. The Middle East and Africa are emerging markets, with rising awareness of hygiene practices and renewable energy initiatives driving incremental ethanol demand.
Conclusion
Although pure ethanol can irritate skin and eyes and cause nausea and vomiting, impure or diluted forms are highly useful. Its effectiveness against a broad spectrum of microorganisms has made it indispensable in the pharmaceutical industry. Ethanol is particularly important in healthcare for hand hygiene and as an antiseptic agent.

Leave a Comment