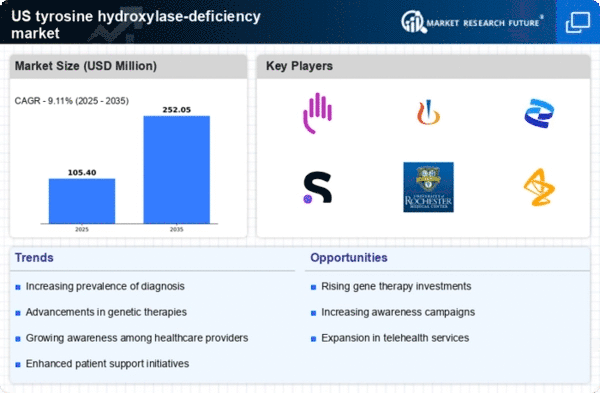
Advancements in Diagnostic Technologies
Technological advancements in diagnostic tools are significantly impacting the tyrosine hydroxylase-deficiency market. Enhanced genetic testing methods, such as next-generation sequencing, allow for more accurate and timely diagnosis of this rare condition. As these technologies become more accessible, healthcare providers can identify affected individuals earlier, which is crucial for effective management and treatment. The market for diagnostic tools is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of around 10% over the next few years. This growth in diagnostic capabilities not only aids in patient management but also encourages pharmaceutical companies to invest in developing targeted therapies, thereby expanding the overall market.
Rising Awareness of Neurological Disorders
The increasing awareness of neurological disorders, including tyrosine hydroxylase deficiency, is driving growth in the tyrosine hydroxylase-deficiency market. Educational campaigns and advocacy efforts by organizations have led to heightened recognition of the symptoms and challenges associated with this condition. As healthcare professionals become more informed, early diagnosis rates are likely to improve, potentially increasing the patient population. This awareness is crucial, as it may lead to more patients seeking treatment options, thereby expanding the market. Furthermore, the emphasis on neurological health in public health discussions suggests a growing commitment to addressing these disorders, which could further stimulate investment in research and development within the tyrosine hydroxylase-deficiency market.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups is playing a pivotal role in the tyrosine hydroxylase-deficiency market. These organizations provide essential resources, education, and community support for individuals and families affected by the condition. By raising awareness and promoting research initiatives, these groups are instrumental in driving demand for effective treatments. Their efforts often lead to increased visibility of the condition, which can influence funding decisions and research priorities. As the number of advocacy groups continues to grow, they are likely to enhance the overall landscape of support for patients, thereby positively impacting the market.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development of innovative therapies in the tyrosine hydroxylase-deficiency market. Initiatives such as orphan drug designations and fast-track approvals are designed to encourage pharmaceutical companies to invest in treatments for rare diseases. This regulatory environment fosters a more favorable landscape for the introduction of new therapies, which could significantly benefit patients. The potential for expedited review processes may lead to quicker access to life-changing treatments, thereby enhancing the market's attractiveness to investors and developers. As regulatory support continues to evolve, it is likely to stimulate growth and innovation within the tyrosine hydroxylase-deficiency market.
Increased Investment in Rare Disease Research
The tyrosine hydroxylase-deficiency market is benefiting from a surge in investment directed towards rare disease research. Government initiatives and private sector funding are increasingly focusing on understanding the underlying mechanisms of rare genetic disorders. This trend is likely to enhance the development of innovative therapies and treatment options for patients. Reports indicate that funding for rare disease research has increased by approximately 15% annually, reflecting a growing recognition of the need for effective treatments. As more resources are allocated to research, the potential for breakthroughs in the tyrosine hydroxylase-deficiency market becomes more promising, potentially leading to new therapeutic avenues.