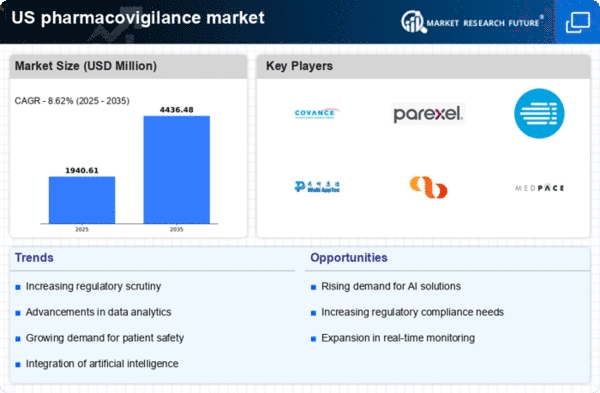
Increasing Regulatory Scrutiny
The pharmacovigilance market is experiencing heightened regulatory scrutiny, particularly in the US. Regulatory bodies such as the FDA are intensifying their focus on drug safety and adverse event reporting. This trend is likely driven by the increasing complexity of drug therapies and the need for robust safety monitoring systems. As a result, pharmaceutical companies are compelled to invest in comprehensive pharmacovigilance systems to ensure compliance with stringent regulations. The market for pharmacovigilance services is projected to grow significantly, with estimates suggesting a CAGR of around 10% over the next few years. This growth reflects the industry's response to regulatory demands and the necessity for effective risk management strategies.
Expansion of Biopharmaceuticals
The rapid expansion of biopharmaceuticals is a key driver of the pharmacovigilance market. As the biopharmaceutical sector continues to grow, the complexity of monitoring the safety of biologics increases. This complexity necessitates enhanced pharmacovigilance practices to ensure the safety and efficacy of these products. Regulatory agencies are placing greater emphasis on the need for rigorous safety monitoring of biopharmaceuticals, which is prompting companies to invest in specialized pharmacovigilance systems. The market is expected to grow in response to this trend, with estimates suggesting a CAGR of around 14% for pharmacovigilance services tailored to biopharmaceuticals over the next several years.
Rising Demand for Patient Safety
Patient safety remains a paramount concern within the pharmacovigilance market. As healthcare providers and patients become more aware of the potential risks associated with medications, there is an increasing demand for effective monitoring systems. This demand is further fueled by the growing prevalence of chronic diseases, which often require long-term medication use. Consequently, pharmaceutical companies are investing in advanced pharmacovigilance solutions to enhance patient safety and ensure timely reporting of adverse events. The market is expected to witness substantial growth, with projections indicating an increase in spending on pharmacovigilance services by approximately 15% in the coming years. This trend underscores the industry's commitment to prioritizing patient welfare.
Growing Focus on Real-World Evidence
The emphasis on real-world evidence (RWE) is reshaping the pharmacovigilance market. Regulatory agencies are increasingly considering RWE in their decision-making processes, which necessitates robust pharmacovigilance systems to collect and analyze data from diverse sources. This trend is particularly relevant in the context of post-marketing surveillance, where understanding the long-term effects of drugs in real-world settings is crucial. Pharmaceutical companies are thus investing in RWE capabilities to enhance their pharmacovigilance efforts. The market is likely to see a surge in demand for services that facilitate the integration of RWE into pharmacovigilance practices, with growth projections indicating an increase of approximately 18% in RWE-related pharmacovigilance services.
Technological Integration in Drug Development
The integration of advanced technologies in drug development is significantly influencing the pharmacovigilance market. As pharmaceutical companies adopt innovative data management solutions, the efficiency of adverse event reporting and analysis improves. Technologies such as cloud computing and big data analytics are becoming essential tools for monitoring drug safety. This shift is likely to enhance the speed and accuracy of pharmacovigilance processes, thereby reducing the time required for regulatory submissions. The market is projected to expand as companies increasingly recognize the value of these technologies, with estimates suggesting a growth rate of around 12% in the adoption of tech-driven pharmacovigilance solutions over the next few years.