Supportive Regulatory Environment
The regulatory landscape for the apheresis market is becoming increasingly favorable, with government agencies providing support for the development and approval of new apheresis technologies. Streamlined approval processes and funding for research initiatives are encouraging innovation within the industry. For example, the FDA has expedited the review of several apheresis devices, which may lead to quicker market entry for new products. This supportive environment is likely to foster competition and drive advancements in apheresis technologies, ultimately benefiting patients and healthcare providers alike. As a result, the apheresis market is expected to expand, with new entrants and established companies alike capitalizing on these regulatory advantages.
Advancements in Apheresis Technology
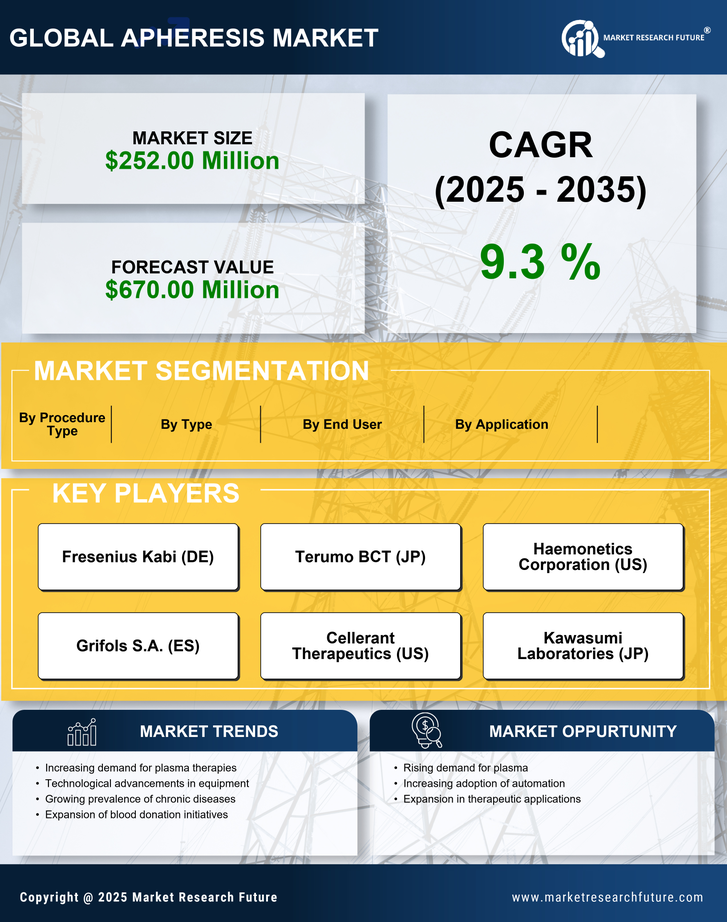
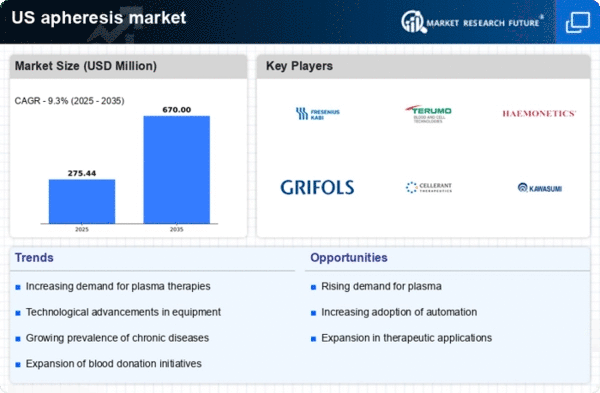
Technological innovations in apheresis equipment and techniques are transforming the apheresis market. The introduction of automated apheresis systems and improved blood component separation technologies enhances the efficiency and safety of procedures. For instance, the latest devices can process blood components with greater precision, reducing the risk of complications. The market for apheresis devices is projected to reach $2 billion by 2026, reflecting a compound annual growth rate (CAGR) of 8%. These advancements not only improve patient care but also expand the range of conditions that can be treated through apheresis, thereby driving growth in the apheresis market.
Growing Awareness of Apheresis Benefits
There is an increasing awareness among healthcare professionals and patients regarding the benefits of apheresis therapies. Educational initiatives and outreach programs are contributing to a better understanding of how apheresis can effectively treat various medical conditions. This heightened awareness is likely to lead to an increase in the number of procedures performed, thereby boosting the apheresis market. As more patients seek alternative treatment options, the industry may experience a shift towards personalized medicine, where apheresis is tailored to individual patient needs. This trend could potentially enhance the market's growth trajectory, as healthcare providers recognize the value of apheresis in comprehensive treatment plans.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as cancer, autoimmune disorders, and hematological conditions is a primary driver for the apheresis market. As these diseases often require therapeutic apheresis procedures, the demand for such treatments is expected to grow. According to recent data, approximately 60% of patients undergoing apheresis are treated for chronic conditions. This trend indicates a significant market opportunity, as healthcare providers increasingly adopt apheresis technologies to manage these diseases effectively. The apheresis market is likely to see a surge in demand for innovative apheresis devices and procedures, as healthcare systems strive to improve patient outcomes and reduce healthcare costs associated with chronic disease management.
Rising Demand for Plasma-Derived Therapies
The increasing demand for plasma-derived therapies is significantly impacting the apheresis market. As the need for immunoglobulins, clotting factors, and other plasma products rises, apheresis procedures become essential for collecting and processing plasma. The market for plasma-derived therapies is projected to grow at a CAGR of 9% over the next five years, indicating a robust demand for apheresis services. This trend is likely to drive investments in apheresis technologies and facilities, as healthcare providers seek to meet the growing needs of patients requiring these life-saving therapies. Consequently, the apheresis market is poised for substantial growth as it adapts to the evolving landscape of plasma therapy.