Regulatory Support and Frameworks
The establishment of supportive regulatory frameworks is a crucial driver for the Stem Cell Manufacturing Market. Regulatory bodies are increasingly recognizing the importance of stem cell therapies and are working to create guidelines that facilitate their development and commercialization. For instance, the U.S. Food and Drug Administration (FDA) has implemented streamlined processes for the approval of regenerative medicine products, which encourages innovation and investment. This regulatory support is essential for ensuring the safety and efficacy of stem cell therapies, thereby instilling confidence among stakeholders. As a result, the Stem Cell Manufacturing Market is likely to benefit from a more favorable environment that promotes research and development activities.
Advancements in Stem Cell Research
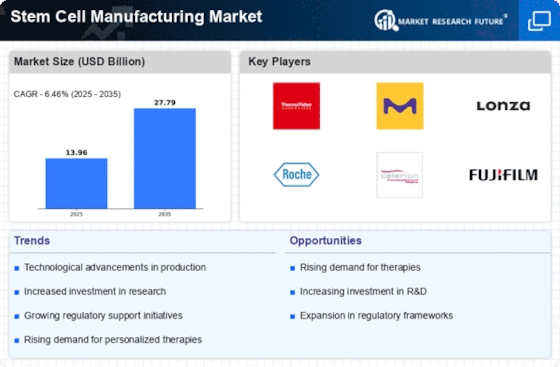
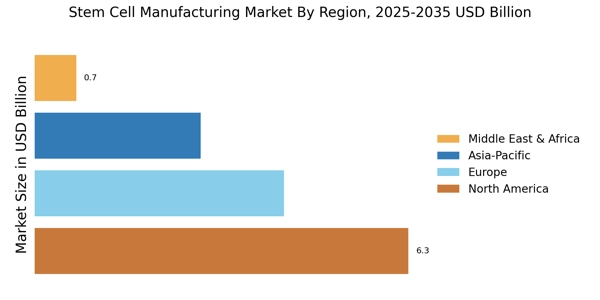
Ongoing advancements in stem cell research significantly influence the Stem Cell Manufacturing Market. Innovations in techniques such as induced pluripotent stem cells (iPSCs) and CRISPR gene editing are revolutionizing the field. These technologies enhance the ability to generate specific cell types for therapeutic purposes, thereby broadening the scope of potential applications. The market is expected to witness a compound annual growth rate (CAGR) of around 10% over the next five years, driven by these scientific breakthroughs. As researchers uncover new insights into stem cell biology, the Stem Cell Manufacturing Market is likely to expand, attracting funding and collaboration opportunities that further propel its development.
Growing Investment in Biotechnology
The surge in investment within the biotechnology sector is a significant driver for the Stem Cell Manufacturing Market. Venture capital funding and public-private partnerships are increasingly directed towards stem cell research and development, reflecting the high potential for returns in this field. In recent years, investments in biotechnology have reached unprecedented levels, with estimates suggesting that funding could exceed 50 billion dollars annually by 2025. This influx of capital not only supports the development of innovative stem cell therapies but also enhances manufacturing capabilities. Consequently, the Stem Cell Manufacturing Market is poised for substantial growth as financial backing enables the exploration of new applications and technologies.
Rising Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases is a critical driver for the Stem Cell Manufacturing Market. Conditions such as cancer, diabetes, and cardiovascular diseases are on the rise, necessitating innovative treatment options. According to recent statistics, chronic diseases account for approximately 70% of all deaths worldwide, underscoring the urgent need for effective therapies. Stem cell-based treatments offer promising solutions for these conditions, leading to heightened interest from healthcare providers and patients alike. As the demand for effective therapies grows, the Stem Cell Manufacturing Market is likely to expand, driven by the need for advanced treatment modalities that address the challenges posed by chronic diseases.
Increasing Demand for Regenerative Medicine
The rising demand for regenerative medicine is a pivotal driver for the Stem Cell Manufacturing Market. As healthcare systems evolve, there is a growing recognition of the potential of stem cells in treating various chronic diseases and injuries. The market for regenerative medicine is projected to reach approximately 100 billion dollars by 2026, indicating a robust growth trajectory. This surge is largely attributed to advancements in stem cell therapies that offer innovative solutions for conditions such as diabetes, heart disease, and neurological disorders. Consequently, the Stem Cell Manufacturing Market is experiencing heightened interest from both investors and researchers, as the therapeutic applications of stem cells continue to expand, fostering a dynamic environment for growth.