

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
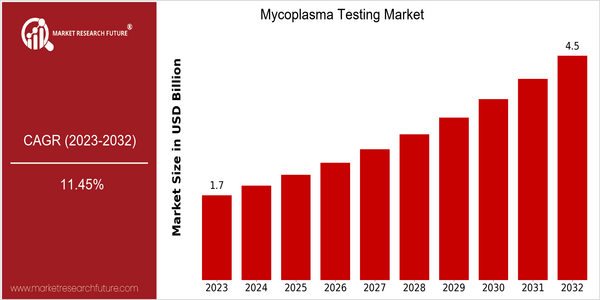
Mycoplasma Testing Market Size Snapshot
| Year | Value |
|---|---|
| 2023 | USD 1.7 Billion |
| 2032 | USD 4.5 Billion |
| CAGR (2024-2032) | 11.45 % |
Note – Market size depicts the revenue generated over the financial year
The mycoplasma test market is estimated to reach $1.8 billion by 2023, and is projected to grow at a CAGR of 11.46% from 2024 to 2032. This significant growth is attributed to the growing demand for mycoplasma testing in various industries, especially in the pharmaceutical, biotechnology, and clinical diagnostics industries. The growing prevalence of mycoplasma contamination in cell cultures and the growing emphasis on quality control in the biopharmaceutical industry are the major driving forces of the mycoplasma test market. Besides, technological advancements such as the development of more sensitive and faster methods are also contributing to the market growth. The development of PCR and NGS techniques has enhanced the sensitivity of mycoplasma detection, thereby improving the reliability of test results. In the near future, the mycoplasma test market will be dominated by Thermo Fisher Scientific, Merck KGaA, and Charles River Laboratories. However, the new entrants such as BioPorto, Gentra, and Gentra BioScience are also expected to gain market shares. Moreover, mycoplasma testing is not only contributing to the growth of the market, but also helping to meet the stringent regulatory requirements.
Regional Deep Dive
The mycoplasma detection market is experiencing significant growth in various regions, owing to the increasing awareness of mycoplasma contamination in cell cultures and the growing need for quality control in biopharmaceutical manufacturing. North America is the largest market, with its advanced health care and stringent regulatory requirements, followed by Europe, with its strong focus on research and development. Asia-Pacific is gaining traction, with its rapid industrialization and increasing investment in biotechnology. Each region has its own unique characteristics, such as the regulatory framework, technological development, and cultural attitudes towards health care.
North America
- The stricter requirements for mycoplasma testing of biologicals have pushed companies to adopt more sophisticated methods of analysis, such as PCR and next-generation sequencing.
- Research and development is being done by companies such as Thermo Fisher Scientific and Merck KGaA to develop new mycoplasma detection kits that improve the accuracy and speed of the tests.
- The increasing frequency of chronic diseases and the growing biopharmaceutical industry in the United States are driving the need for reliable mycoplasma testing solutions.
Europe
- A new mycoplasma testing protocol introduced by the EMA will affect the growth of the cell therapy market.
- BioMérieux and Charles River Laboratories have developed new products for the European market.
- The pronounced focus on biopharmaceutical innovation and the high quality requirements are creating a highly competitive environment for mycoplasma testing.
Asia-Pacific
- In the last few years, the biotechnology industry in India and China has grown at an impressive rate, resulting in a growing demand for mycoplasma testing in both research and production.
- The introduction of new government initiatives aimed at promoting the region’s biomanufacturing capacity is driving the adoption of mycoplasma testing solutions.
- The development of cheap and easy-to-use mycoplasma detection kits is a priority for local companies.
MEA
- The Middle East and Africa are seeing a growing interest in the production of biopharmaceuticals, which are increasingly dependent on reliable mycoplasma testing to ensure the safety of the product.
- Regulatory authorities in countries such as South Africa are beginning to develop guidelines for mycoplasma testing that will be a model for the whole region.
- Between the local universities and the international biotechnology companies, new methods of mycoplasma detection are being developed.
Latin America
- Latin America is gradually adopting mycoplasma testing because of an increased awareness of the risks of contamination in biopharmaceutical production.
- Brazil, for example, has recently passed regulations that encourage local manufacturers to carry out stringent quality controls, including mycoplasma tests.
- Local and international research institutions are working together to share knowledge and technology in the field of mycoplasma testing.
Did You Know?
“Mycoplasma contaminations are responsible for about 30 per cent of contaminations of cell cultures, and have a significant impact on research results and product quality.” — Journal of Biotechnology
Segmental Market Size
The Mycoplasma Assay Market is currently growing steadily, driven by the increasing demand for accurate and fast diagnostics in various industries, particularly in pharmaceuticals and biotechnology. Regulatory authorities have tightened their testing requirements for biologicals and the growing occurrence of mycoplasma contamination in cell cultures has increased the demand for effective testing solutions. Advances in molecular diagnostics are also driving the market.
Among the firms which have taken the lead in the development of the mycoplasma test are Thermo Fisher Scientific and Roche. Their main applications are in the quality control of vaccine production and cell culture, where mycoplasma contamination can cause the products to be of very poor quality. These are the most demanding areas of use. The pandemic of H1N1 has accentuated the need for stricter testing procedures. Moreover, government regulations relating to the quality control of biopharmaceuticals have lent momentum to the market. The development of the market is being driven by PCR and next-generation sequencing, which enable faster and more reliable testing procedures to be developed.
Future Outlook
The Mycoplasma Testing Market is estimated to grow at a robust CAGR of 11.36% from 2023 to 2032, with an increase in the projected market size from $1.7 billion to $4.5 billion. This growth is primarily driven by the increasing demand for mycoplasma testing in the pharmaceutical, biotechnology, and clinical diagnostics industries. In the coming years, the regulatory authorities are expected to continue to lay greater emphasis on the quality control of biologicals and cell-based products. Consequently, it is estimated that more than 60% of biopharmaceutical companies will integrate mycoplasma testing into their quality assurance (QA) programs by 2032, which will further increase the importance of these tests for ensuring the safety and efficacy of products.
This is the most important part of the market. The new developments in PCR and next-generation sequencing are making it more accurate and efficient to detect mycoplasma. And the development of cell and gene therapies is bringing new and more complex testing solutions. The market will develop, and the main players will probably invest in R & D to develop new methods of testing with faster turn-around times and increased sensitivity. In addition, the growing focus on regulatory compliance and the need for rapid diagnostics to cope with the growing number of diseases and health problems will further drive the market and put mycoplasma analysis in the center of the broader health care landscape.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 16.45% |
Mycoplasma Testing Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









