

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
Montelukast API Market Size Snapshot
| Year | Value |
|---|---|
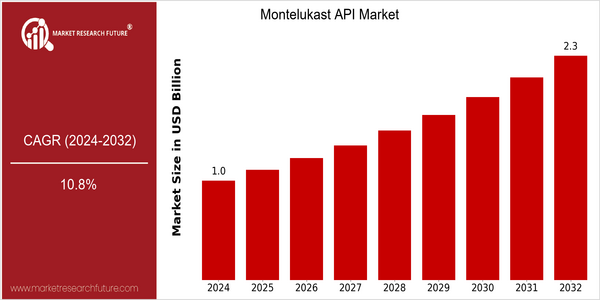
| 2024 | USD 1.01 Billion |
| 2032 | USD 2.3 Billion |
| CAGR (2024-2032) | 10.8 % |
Note – Market size depicts the revenue generated over the financial year
Montelukast is the active ingredient in the drug Montelukast. The Montelukast API market is expected to grow at a CAGR of 15.3 per cent from 2024 to 2032. This growth is a result of the increasing demand for the drug in the emerging economies. The increasing prevalence of asthma and other rhinitis is expected to drive the demand for the drug. Moreover, advancements in the drug formulation and delivery systems are enhancing the efficacy and patient compliance of the drug. The leading companies in the Montelukast API market, such as Merck & Co., Inc. and Teva Pharmaceutical Industries Ltd., are focusing on the development of new formulations to capitalize on the market growth. The companies are also focusing on expanding their production capacities and establishing strategic alliances to strengthen their market presence. Several collaborations to improve the supply chain efficiencies and enhance the patient access to the drug are expected to further intensify the competition. As the market evolves, innovation in the drug development and a focus on the patient-centric solutions are expected to shape the future of the Montelukast API market.
Regional Deep Dive
The Montelukast market is characterized by a growing demand for asthma and allergy drugs, mainly owing to an increasing number of cases of airway diseases and a growing awareness of preventive medicine. In North America, the market is characterized by a highly developed health care system and substantial investments in pharmaceutical R & D. Europe is characterized by a strong regulatory framework, which promotes innovation and ensures patient safety. The Asia-Pacific region is characterized by a rapid growth due to urbanization and changing lifestyles, which lead to a higher prevalence of asthma and allergies. The Middle East and Africa (MEA) region is characterized by its unique challenges, such as the different access to health care and the regulatory framework, while the Latin America region is characterized by a gradual increase in the penetration of health care systems.
North America
- The U.S. Food and Drug Administration has recently shortened the approval process for generic versions of montelukast. This is expected to increase competition in the market and reduce the cost of the drug.
- The leading companies like Merck & Co. are investing in the development of new formulations of Montelukast with the aim of improving patient compliance and reducing side effects.
- In the United States, the prevalence of asthma in children is increasing, causing a rise in demand for montelukast.
Europe
- The European Medicines Agency (EMC) has issued stricter guidelines for the approval of montelukast, stressing the need for comprehensive safety data, especially on neuropsychiatric effects.
- To make the drug more effective, Teva has studied the possibility of delivering it by inhalation.
- The increasing trend towards individualized medicine in Europe is influencing the development of monteleukast products tailored to the different patient groups, in particular in order to meet the varying response to treatment.
Asia-Pacific
- Asthma cases are rising in India and China.
- In view of the growing demand, the local manufacturers are increasing their production capacity. Sun Pharmaceuticals Industries is leading the race in the generic montelukast market.
- The market for Montelukast is expected to grow, especially in rural areas, with the implementation of government initiatives to improve access to and the cost of medical care.
MEA
- The World Health Organization has launched a programme to improve the management of asthma in the region. Its programme includes the promotion of the use of Montelukast as the first-line treatment.
- The distribution of Montelukast in the Middle East and Africa region is restricted by the regulatory differences between countries. Some countries require extensive clinical data for approval.
- The knowledge about asthma is spreading and the acceptance of Montelukast as a treatment is growing.
Latin America
- Brazil has launched a program of subsidies to reduce the cost of asthma medication, including Montelukast, to make it more accessible to the lower-income population.
- In the field of pharmaceuticals, Latin America is becoming a more and more important market, and there is a growing trend of companies to invest in the region in order to reduce costs and improve supply chains.
- Allergic rhinitis, a prevailing disease in the cities, has made the use of Montelukast necessary. The medical profession has recommended it more frequently.
Did You Know?
“Montelukast was first approved by the U.S. Food and Drug Administration in 1998, and has since become one of the most widely prescribed drugs for asthma and allergic rhinitis.” — FDA Approval History
Segmental Market Size
Montelukast API is an important part of the respiratory treatment market, which is currently experiencing a steady growth. There are several factors that are driving this market, such as the increasing prevalence of asthma and allergic rhinitis, which necessitate effective treatment. Moreover, there is a growing awareness of the importance of chronic respiratory diseases amongst both health care professionals and patients. This is especially true in emerging economies, where access to medical care is improving.
The development of the Montelukast a.p. is now in its final stage, and several leading pharmaceutical companies, such as Merck and Teva, are already producing and distributing the substance. The first use of the substance is in the form of tablets and chewables for children with asthma and seasonal allergies. The development of this field is stimulated by the growing emphasis on individualized medicine and the integration of digital health solutions. Also, new formulations, such as controlled-release systems, are improving the effectiveness and patient compliance of Montelukast-based therapies.
Future Outlook
The market for Montelukast is expected to grow significantly between 2024 and 2032, from $1.01 billion to $2.3 billion, at a robust CAGR of 10.8%. The escalation in asthma and allergic rhinitis is expected to increase the demand for effective treatments. As a result, the penetration of Montelukast in the treatment of these conditions will increase, according to industry forecasts and epidemiological studies, reaching more than 30% in patients with chronic respiratory diseases by 2032.
The main technological advances in drug formulation and delivery systems will continue to develop the market. Personalization and combination therapies, which improve patient outcomes and adherence, will also support the adoption of montelukast. Furthermore, supportive regulatory policies, which increase access to essential medicines, will play a critical role in market growth. The increasing focus on preventive medicine and the integration of digital health solutions will also drive the market, with montelukast becoming a core treatment for the management of respiratory diseases over the next decade.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Market Size Value In 2022 | USD 0.8 Billion |
| Market Size Value In 2023 | USD 0.9 Billion |
| Growth Rate | 12.50% (2023-2032) |
Montelukast API Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









