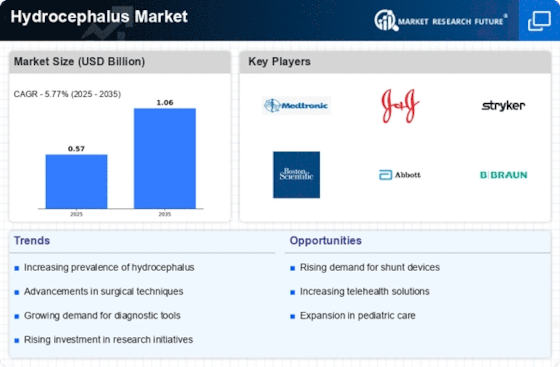
Top Industry Leaders in the Hydrocephalus Market
 Latest Hydrocephalus Companies Update
Latest Hydrocephalus Companies Update
-
Sep 2023: According to a release, the FDA has given 510(k) approval to a radiology artificial intelligence company to triage and notify patients with obstructive hydrocephalus on non-contrast brain CT scans. According to a press release from Annalise.ai, the FDA also designated an obstructed hydrocephalus software tool as a breakthrough product. The FDA's Breakthrough Devices Program was established to identify medical devices that enhance the treatment or diagnosis of dangerous or debilitating conditions, intending to facilitate faster access to these devices for healthcare providers. According to the release, this clearance represents the first time a radiology triage has been granted breakthrough status. According to the business, Annalise.ai's solution enables active and passive warnings for suspected OHCP instances found on unenhanced head CT images. The cerebrospinal fluid that has accumulated in the cranial vault can cause obstructive hydrocephalus, a potentially fatal illness that can worsen quickly if treatment is not received.
-
Oct 2023: With the help of its eShunt implant, CereVasc has now treated thirty patients. The operation is a component of the company's less invasive therapy for communicating hydrocephalus, which is the subject of continuing pilot studies in the US and Argentina. Patients with communicative hydrocephalus who are unable to wean themselves off of an external ventricular drain after a hemorrhagic episode are the subjects of the pilot trials conducted in the US and Argentina. The subjects will undergo evaluations each month for ninety days following implantation. After that, post-operative check-ups will be after 180 days, 12 months, and 24 months. The main result is a decrease in intracranial pressure.
List of Hydrocephalus Key companies in the market
- DePuy Synthes
- Boston Neurosciences
- Medtronic Plc
- Integra Life Sciences Corporation
- Spiegelberg GmbH & Co. KG
- RAUMEDIC Inc
- Sophysa SA
- Terumo Corporation
- Natus Medical Incorporated
- Johnson & Johnson Services Inc