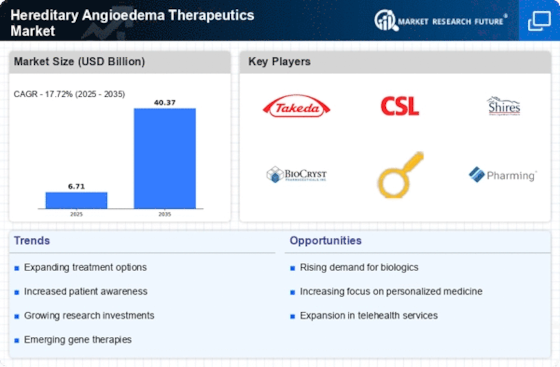
Top Industry Leaders in the Hereditary Angioedema Therapeutics Market

Latest Hereditary Angioedema Therapeutics Companies Update:
BioCryst Pharmaceuticals Received FDA approval for Orladeyo™ (berotralstat) for prophylaxis of HAE attacks, offering the first and only once-daily oral medication for this indication.Announced positive late-stage clinical trial results for beronalstat in pediatric HAE patients, potentially expanding its market reach.
Takeda Pharmaceutical Company Launched Takhzyro™ (lanadelumab) in the US, providing a long-acting subcutaneous injection for HAE prophylaxis.Initiated Phase 3 clinical trials for its novel C5a receptor inhibitor TAK-994 for HAE prevention, expanding its treatment pipeline.
Shire Received FDA approval for Cinryze™ (C1 esterase inhibitor) for the treatment of acute HAE attacks, offering a rapid-acting intravenous option.Collaborated with patient advocacy groups to launch educational initiatives and improve access to HAE care.
Alexion Pharmaceuticals Continued to be a leader in the HAE market with its established medications Soliris™ (eculizumab) and Ultomiris™ (ravulizumab), offering long-term prevention and treatment options. Focused on expanding access to its therapies through patient assistance programs and advocacy partnerships.
List of Hereditary Angioedema Therapeutics Key companies in the market
- Takeda Pharmaceutical Company Limited (Japan)
- CSL Behring (US)
- Sanofi (France)
- BioCryst Pharmaceuticals, Inc (US)
- Pharvaris B.V. (Switzerland)
- Ionis Pharmaceuticals, Inc (US)
- Pharming Group N.V. (Netherlands)
- KalVista Pharmaceuticals, Inc (US)
- IBio, Inc. (US)
- BioMarin Pharmaceutical Inc (US)