GCC Sterility Testing Market Summary
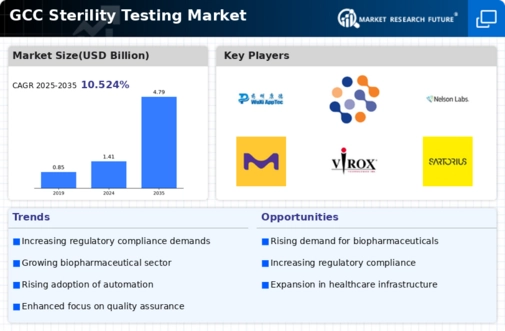
The GCC Sterility Testing Market is projected to grow significantly from 1.41 USD Billion in 2024 to 4.79 USD Billion by 2035.
Key Market Trends & Highlights
GCC Sterility Testing Market Key Trends and Highlights
- The GCC Sterility Testing Market is valued at 1.41 USD Billion in 2024.
- By 2035, the market is expected to reach 4.79 USD Billion, indicating robust growth.
- The market is anticipated to grow at a CAGR of 11.76% from 2025 to 2035.
- Growing adoption of advanced testing technologies due to increasing regulatory requirements is a major market driver.
Market Size & Forecast
| 2024 Market Size | 1.41 (USD Billion) |
| 2035 Market Size | 4.79 (USD Billion) |
| CAGR (2025-2035) | 11.76% |
Major Players
Wuxi AppTec, Eurofins Scientific, Nelson Labs, Ebike Technologies, Merck KGaA, Virox Technologies, Sartorius, BioMérieux, Avantor, Charles River Laboratories, MilliporeSigma, 21st Century Biochemicals, BD, Microtest Labs, Thermo Fisher Scientific