North America : Market Leader in Facial Treatments
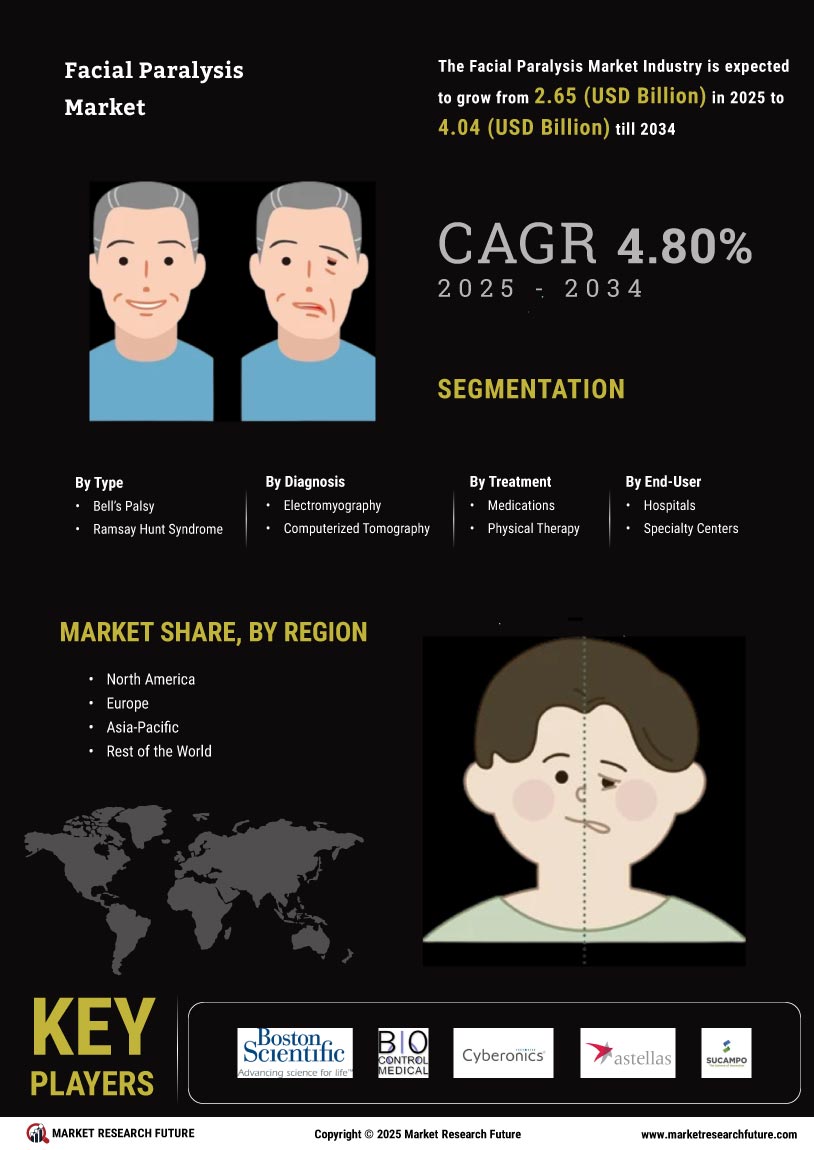
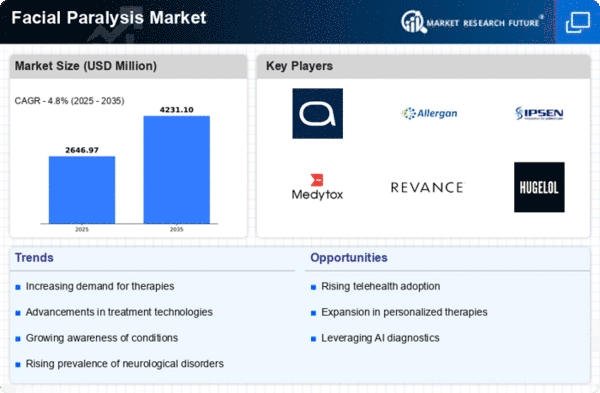
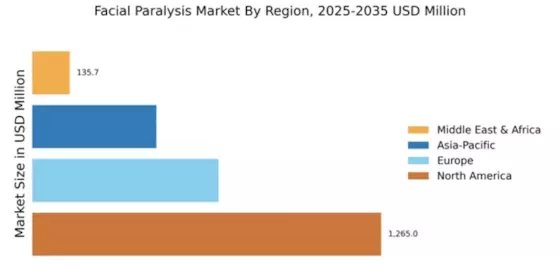
North America is poised to maintain its leadership in the Facial Paralysis Market, holding a significant market share of $1265.0M in 2024. The region's growth is driven by increasing awareness of facial paralysis treatments, advancements in medical technology, and supportive regulatory frameworks. The demand for innovative therapies, including botulinum toxin injections and surgical interventions, is on the rise, fueled by a growing aging population and higher incidences of neurological disorders. The competitive landscape in North America is robust, featuring key players such as AbbVie, Allergan, and Revance Therapeutics. These companies are at the forefront of developing cutting-edge treatments, enhancing patient outcomes. The presence of well-established healthcare infrastructure and favorable reimbursement policies further bolster market growth. As the region continues to innovate, it is expected to attract significant investments, solidifying its position as a market leader.
Europe : Emerging Market with Growth Potential
Europe is witnessing a growing interest in the Facial Paralysis Market, with a market size of $675.0M in 2024. The region's growth is driven by increasing healthcare expenditure, rising awareness of treatment options, and a focus on improving patient quality of life. Regulatory bodies are actively promoting research and development in this field, which is expected to catalyze market expansion. The demand for minimally invasive procedures is also on the rise, contributing to market dynamics. Leading countries in Europe, such as Germany, France, and the UK, are key players in this market. The competitive landscape includes major companies like Ipsen and Galderma, which are investing in innovative therapies. The presence of a well-established healthcare system and supportive regulations enhances the market environment. As Europe continues to embrace advancements in treatment options, it is set to become a significant player in the global market.
Asia-Pacific : Rapidly Growing Market Opportunities
The Asia-Pacific region is emerging as a significant player in the Facial Paralysis Market, with a market size of $450.0M in 2024. The growth is driven by increasing healthcare access, rising disposable incomes, and a growing awareness of facial paralysis treatments. Regulatory support for new therapies and a focus on enhancing healthcare infrastructure are also contributing to market dynamics. The demand for effective treatment options is expected to rise, particularly in urban areas. Countries like South Korea, Japan, and Australia are leading the charge in this market. Key players such as Medytox and Hugel are actively involved in developing innovative solutions. The competitive landscape is characterized by a mix of local and international companies, fostering a dynamic environment. As the region continues to evolve, it is likely to attract investments and partnerships, further enhancing its market position.
Middle East and Africa : Emerging Market with Unique Challenges
The Middle East and Africa region is gradually developing its Facial Paralysis Market, with a market size of $135.73M in 2024. The growth is influenced by increasing healthcare investments, rising awareness of treatment options, and a focus on improving healthcare access. However, challenges such as regulatory hurdles and varying healthcare infrastructure across countries may impact market growth. The demand for effective treatments is expected to rise as awareness increases. Leading countries in this region include South Africa and the UAE, where healthcare systems are evolving. The competitive landscape features both local and international players, with companies exploring opportunities to introduce innovative therapies. As the region continues to develop, it is likely to see increased collaboration and investment in healthcare solutions, enhancing its market potential.