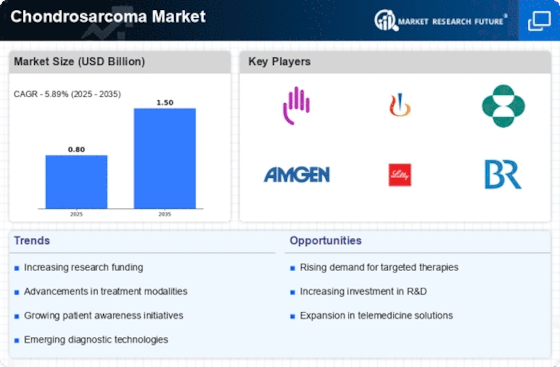
Top Industry Leaders in the Chondrosarcoma Market
 Latest Chondrosarcoma Companies Update
Latest Chondrosarcoma Companies Update
-
April 2023: Following the lifting of a partial clinical halt on two trials by the US Food and Drug Administration (FDA), Inhibrx has successfully resumed its chondrosarcoma program. Inhibrx declared that patient recruitment for a Phase II and Phase I trial evaluating INBRX-109 in uncommon bone cancers would recommence the following month. Due to concerns regarding liver toxicity, the trials were halted, and Inhibibrx subsequently modified the study protocols to exclude patients at risk. The Hepatic Steatosis Index (HSI), a metric utilized in both trials for screening purposes, evaluates the severity of fatty liver diseases in order to exclude geriatric participants who are particularly susceptible to severe liver toxicity. Pending enrollment was declared by Inhibrx in March 2023 in accordance with predetermined cessation criteria; patients who were already undergoing treatment were not impacted by the move. The registration-enabling Phase II trial of INBRX-109 (NCT04950075) is involving 201 patients with metastatic or unresectable conventional chondrosarcoma.
-
August 2022: In response to the European Medicines Agency's (EMA) favorable assessment, the European Commission (EC) has conferred Inhibrx, Inc. orphan medicinal product designation for chondrosarcoma treatment INBRX-109. Orphan designation is bestowed by the European Commission (EC) upon pharmaceuticals and biologics that are developed for the safe and efficient treatment, prevention, or diagnosis of a chronically debilitating disease that affects fewer than five out of every 10,000 patients within the European Union. The European Union (EU) may grant specific advantages to orphan drugs, such as reduced regulatory fees, support with clinical protocols, and the possibility of ten years of market exclusivity subsequent to regulatory approval.
List of Chondrosarcoma Key companies in the market
- Abbott. (US)
- AbbVie Inc. (US)
- Akorn, Inc. (US)
- Agios, Inc. (US)
- Baxter (US)
- Bayer AG (US)
- Epizyme, Inc. (US)
- Novartis AG (Switzerland)
- Mylan N.V. (US)