Market Growth Projections
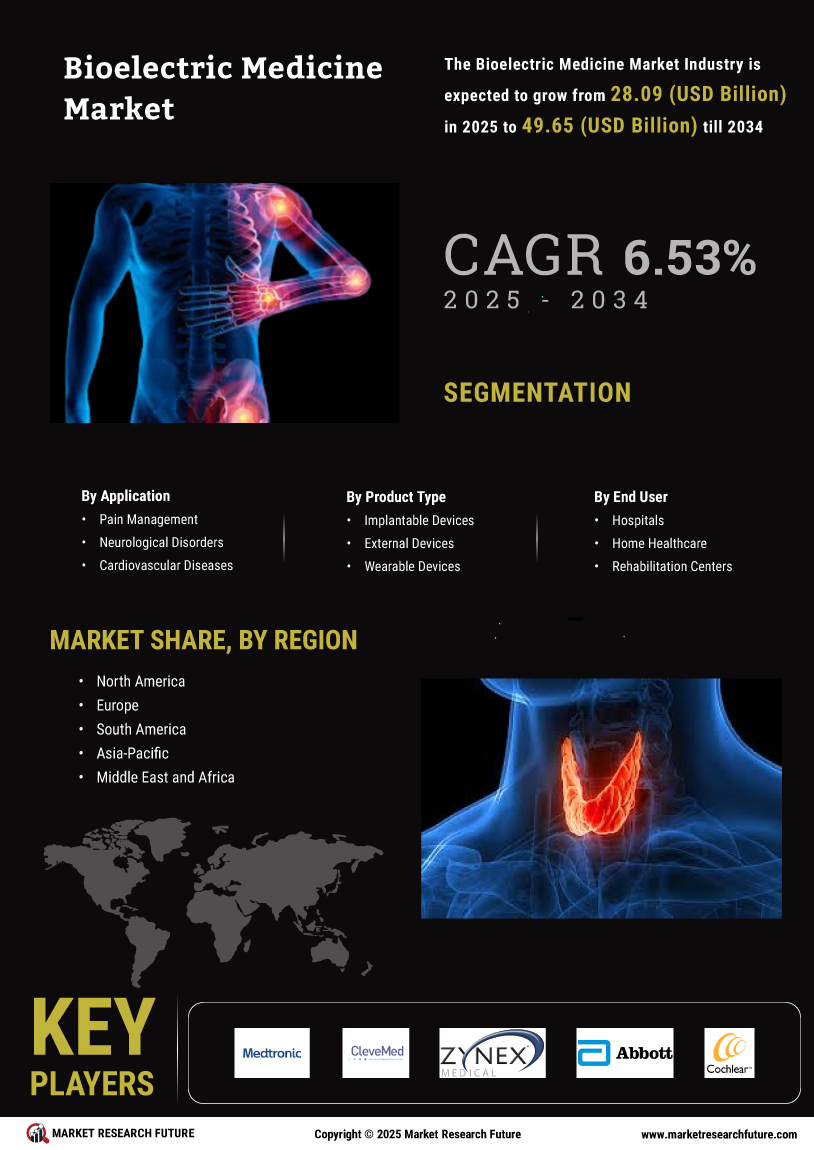
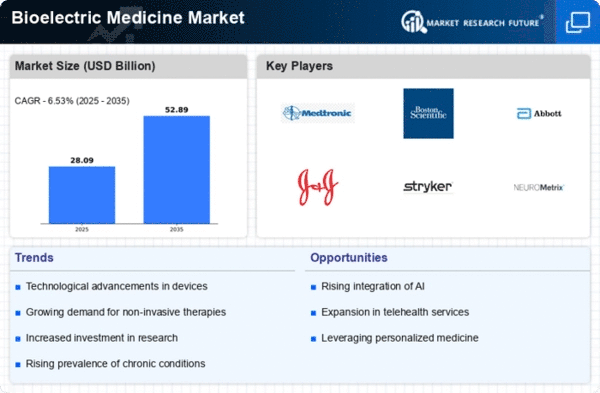
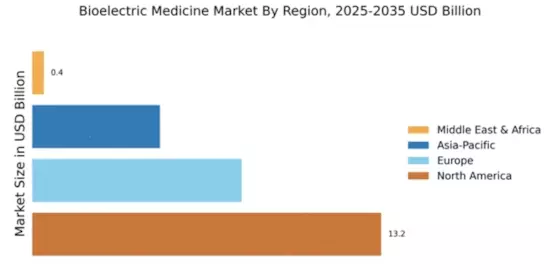
The Global Bioelectric Medicine Market Industry is poised for substantial growth, with projections indicating a market value of 26.4 USD Billion in 2024. By 2035, this figure is expected to reach 52.9 USD Billion, reflecting a compound annual growth rate of 6.53% from 2025 to 2035. This growth is driven by various factors, including the rising prevalence of chronic diseases, technological advancements in medical devices, and increasing demand for non-invasive treatment options. The market's expansion is indicative of the growing recognition of bioelectric medicine as a viable therapeutic approach, likely leading to further innovations and investments in the sector.
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases globally is a primary driver of the Global Bioelectric Medicine Market Industry. Conditions such as diabetes, cardiovascular diseases, and neurological disorders necessitate innovative treatment solutions. Bioelectric medicine, which utilizes electrical impulses to modulate biological functions, offers promising therapeutic options. For instance, the World Health Organization reports that chronic diseases account for 71% of all deaths worldwide. This trend is expected to propel the market, with projections indicating a market value of 26.4 USD Billion in 2024, potentially reaching 52.9 USD Billion by 2035, reflecting a compound annual growth rate of 6.53% from 2025 to 2035.
Technological Advancements in Medical Devices
Technological innovations in medical devices are significantly influencing the Global Bioelectric Medicine Market Industry. Advancements in microelectronics, wireless technology, and biocompatible materials have led to the development of sophisticated bioelectric devices. These devices, such as neurostimulators and implantable cardioverter-defibrillators, are becoming more effective and user-friendly. For example, the integration of artificial intelligence in bioelectric devices enhances their functionality and adaptability. As these technologies evolve, they are expected to attract more investment and research, thereby expanding the market. The anticipated growth trajectory suggests that the market could see a valuation of 52.9 USD Billion by 2035.
Aging Population and Associated Healthcare Needs
The global aging population is a significant driver of the Global Bioelectric Medicine Market Industry. As individuals age, they often experience a higher prevalence of chronic conditions that require effective management. Bioelectric medicine provides innovative solutions for age-related ailments, such as pain management and neurological disorders. The United Nations projects that the number of people aged 60 and older will reach 2.1 billion by 2050, creating a substantial demand for bioelectric therapies. This demographic shift is likely to contribute to the market's growth, with expectations of reaching 26.4 USD Billion in 2024 and potentially doubling by 2035.
Growing Demand for Non-Invasive Treatment Options
The shift towards non-invasive treatment modalities is a notable driver of the Global Bioelectric Medicine Market Industry. Patients increasingly prefer therapies that minimize surgical risks and recovery times. Bioelectric medicine offers non-invasive alternatives for pain management and rehabilitation, appealing to both patients and healthcare providers. For instance, transcutaneous electrical nerve stimulation (TENS) units are widely used for pain relief without the need for invasive procedures. This growing demand for safer treatment options is likely to enhance market growth, with projections indicating a market size of 26.4 USD Billion in 2024, potentially doubling by 2035.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a crucial factor propelling the Global Bioelectric Medicine Market Industry. Governments and private sectors are allocating substantial resources to enhance healthcare facilities and technology. This investment facilitates the adoption of advanced bioelectric medical devices and therapies. For example, initiatives aimed at improving access to healthcare in developing regions are likely to increase the demand for bioelectric solutions. As healthcare systems evolve, the market is expected to grow, with estimates suggesting a valuation of 52.9 USD Billion by 2035, driven by enhanced healthcare delivery and technology integration.