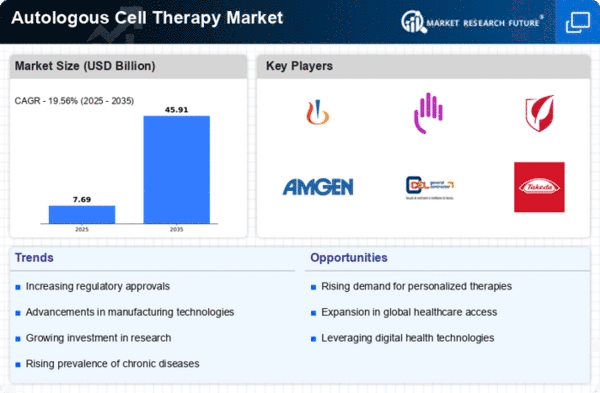
Top Industry Leaders in the Autologous Cell Therapy Market

Latest Autologous Cell Therapy Companies Update:
December 2023:
AtCure Pharmaceuticals partners with Cytovia on Phase IIB for APL1301 for advanced solid tumor. This signifies progress in broadening autologous cell therapy applications for cancer.
November 2023:
Fate Therapeutics and Takeda Pharmaceuticals International collaborate on development and commercialization of CAR T-cell therapy for CD30+ lymphomas. This highlights the continued focus on expanding CAR T-cell therapy beyond B-cell malignances.
Jan 2024:
Legend Biotech receives China National Medical Products Administration (NMPA) approval for LCAR-017 for relapsed/refractory B-cell lymphomas. This signifies the first regulatory approval for CAR T-cell therapy in China.
Dec 2023:
BlueRock Therapeutics releases encouraging Phase I/II data for autologous T-cell therapy for PD-L1 negative non-small cell lung cancer. this marks progress in tackling challenging cancer types with autologous therapies.
Jan 2024:
Report projects autologous cell therapy market to reach $33.1 billion by 2032, fueled by rising demand for personalised treatments. This underscores the market's continued strong growth potential.
Oct 2023:
Thermo Fisher and UCSF unveil new cGMP cell therapy manufacturing facility. This highlights ongoing efforts to overcome manufacturing challenges and expedite therapy development.
List of Autologous Cell Therapy Key companies in the market
- Bristol Myers Squibb (US)
- Bayer AG (Germany)
- Autolus Therapeutics (UK)
- Sangamo Therapeutics (US)
- Holostem Terapie Avanzate S.r.l.(Modena)
- Vericel Corporation (US)
- Opexa Therapeutics (US)
- BrainStorm Cell Therapeutics (US)
- Pharmicell Co., Inc. (South Korea)
- Daiichi Sankyo Co (Japan)., Ltd, among others