-
Report Prologue
-
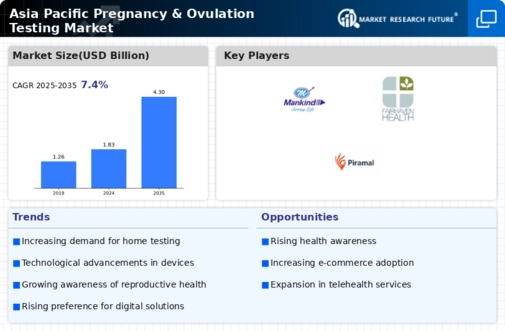
Executive Summary
-
Market Introduction
-
Definition 15
-
Scope Of The Study 15
-
Assumptions & Limitations 15
- Assumptions 15
- Limitations 15
-
Market Structure 16
-
Research Methodology
-
Research Process 18
-
Primary Research 19
-
Secondary Research 20
-
Market Size Estimation 21
-
Market Dynamics
-
Introduction 23
-
Drivers 24
- Declining Fertility Rate 24
- Emergence Of Advanced Technology With High Accuracy 24
- Easy Availability Of Ovulation & Pregnancy Monitors On E-Commerce Websites 25
-
Restraints 25
- High Cost Associated With Ovulation Tests 25
-
Opportunities 25
- Growing Awareness About Pregnancy And Ovulation Testing 25
-
Market Factor Analysis
-
Porter’s Five Forces Analysis 27
- Bargaining Power Of Suppliers 27
- Bargaining Power Of Buyers 28
- Threat Of New Entrants 28
- Threat Of Substitutes 28
- Intensity Of Rivalry 28
-
Supply Chain Analysis 29
- R&D 29
- Parts Manufacturing 29
- Distribution 29
- Marketing & Sales 29
- Post-Sales Monitoring 30
-
Pricing Analysis 30
-
Investment Opportunities 30
-
Asia Pacific Pregnancy & Asia Pacific Pregnancy Ovulation Testing Market, By Product Type
-
Overview 32
- Introduction 32
- Pregnancy Testing (HCG Detection) 33
- Ovulation Testing 36
-
Asia Pacific Pregnancy & Asia Pacific Pregnancy Ovulation Testing Market, By Mode Of Purchase
-
Overview 40
- Introduction 40
- Non-Prescription Or OTC Testing 41
- Prescription Testing 42
-
Asia Pacific Pregnancy & Asia Pacific Pregnancy Ovulation Testing Market, By End-User
-
Overview 44
- Introduction 44
- Hospitals & Clinics 45
- Fertility Centres 46
- Others 46
-
Asia Pacific Pregnancy & Asia Pacific Pregnancy Ovulation Testing Market, By Country/Region
-
Asia Pacific 48
- Japan 51
- China 53
- India 55
- Republic Of Korea 57
- Australia 59
- Rest Of Asia Pacific 61
-
Competitive Landscape
-
Introduction 64
-
Company Share Analysis 64
-
Company Profiles
-
SPD Swiss Precision Diagnostics GmbH 66
- Company Overview 66
- Financial Overview 66
- Products Offering 67
- Key Developments 67
- SWOT Analysis 67
- Key Strategy 68
-
Fairhaven Health, LLC 69
- Company Overview 69
- Financial Overview 69
- Products Offering 69
- Key Developments 69
- SWOT Analysis 70
- Key Strategy 70
-
Church & Dwight Co., Inc. 71
- Company Overview 71
- Financial Overview 71
- Products Offering 72
- Key Developments 72
- SWOT Analysis 72
- Key Strategy 72
-
Piramal Enterprises Ltd. 73
- Company Overview 73
- Financial Overview 73
- Products Offering 74
- Key Developments 74
- SWOT Analysis 74
- Key Strategy 74
-
Quidel Corporation 75
- Company Overview 75
- Financial Overview 75
- Products Offering 76
- Key Developments 76
- SWOT Analysis 76
- Key Strategy 76
-
Mankind Pharma Ltd. 77
- Company Overview 77
- Financial Overview 77
- Products Offering 78
- Key Developments 78
- SWOT Analysis 78
- Key Strategy 78
-
TaiDoc Technology & Corporation 79
- Company Overview 79
- Financial Overview 79
- Products Offering 79
- Key Developments 80
- SWOT Analysis 80
- Key Strategy 80
-
Cadila Healthcare Limited 81
- Company Overview 81
- Financial Overview 81
- Products Offering 82
- Key Developments 82
- SWOT Analysis 82
- Key Strategy 82
-
Gregory Pharmaceutical Holdings, Inc. 83
- Company Overview 83
- Financial Overview 83
- Products Offering 83
- Key Developments 83
- SWOT Analysis 83
- Key Strategy 84
-
Appendix
-
Discussion Blue Print 86
-
References 87
-
List Of Tables
-
MARKET SYNOPSIS 13
-
PRIMARY INTERVIEWS 18
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET BY PRODUCT TYPE, 2023-2032 (USD MILLION) 33
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET FOR PREGNANCY TESTING (HCG DETECTION), BY TYPE, 2023-2032 (USD MILLION) 33
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET FOR PREGNANCY TESTING (HCG DETECTION), BY COUNTRY/REGION, 2023-2032 (USD MILLION) 34
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 35
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 35
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 36
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 37
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 37
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 38
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 38
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 41
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 41
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 42
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET BY END-USER, 2023-2032 (USD MILLION) 45
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET FOR HOSPITALS & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 45
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 46
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023-2032 (USD MILLION) 49
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 49
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 49
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 50
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 50
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 50
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 50
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 51
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 51
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 51
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 51
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 52
-
JAPAN: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 52
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 53
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 53
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 53
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 53
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 54
-
CHINA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 54
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 55
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 55
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 55
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 55
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 56
-
INDIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 56
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 57
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 57
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 57
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 58
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 58
-
REPUBLIC OF KOREA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 58
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 59
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 59
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 59
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 59
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 60
-
AUSTRALIA: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 60
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023-2032 (USD MILLION) 61
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PREGNANCY TESTING TYPE, 2023-2032 (USD MILLION) 61
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY OVULATION TESTING TYPE, 2023-2032 (USD MILLION) 61
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY TYPE, 2023-2032 (USD MILLION) 62
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023-2032 (USD MILLION) 62
-
REST OF ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023-2032 (USD MILLION) 62
-
List Of Figures
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET: MARKET STRUCTURE 16
-
RESEARCH PROCESS 18
-
TOP-DOWN & BOTTOM-UP APPROACH 21
-
MARKET DYNAMICS ANALYSIS OF ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET 23
-
PORTERS FIVE FORCES ANALYSIS OF ASIA PACIFIC PREGNANCY AND OVULATION TESTING MARKET 27
-
SUPPLY CHAIN: ASIA PACIFIC PREGNANCY AND OVULATION TESTING MARKET 29
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023 (% SHARE) 32
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY PRODUCT TYPE, 2023 & 2032 (USD MILLION) 32
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023 (% SHARE) 40
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY MODE OF PURCHASE, 2023 & 2032 (USD MILLION) 40
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023 (% SHARE) 44
-
ASIA PACIFIC PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY END-USER, 2023 & 2032 (USD MILLION) 44
-
ASIA PACIFIC: PREGNANCY & Asia Pacific Pregnancy Ovulation Testing Market, BY COUNTRY/REGION, 2023 (%) 48
-
ASIA PACIFIC PREGNANCY & OVULATION TESTING MARKET, MARKET SHARE ANALYSIS 2023 (%) 64














Leave a Comment