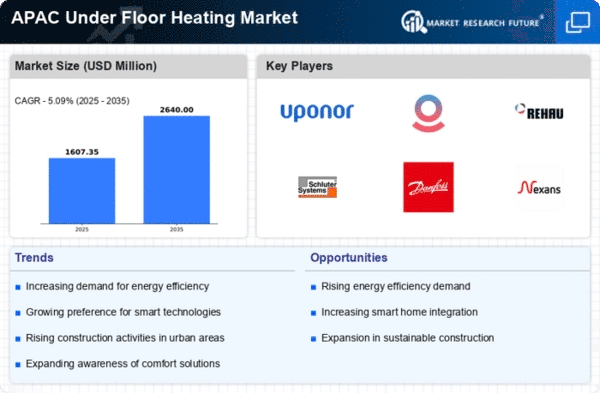
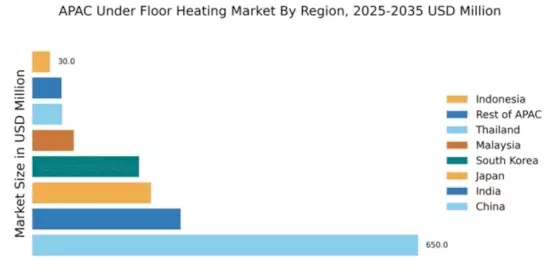
Increasing Urbanization
The rapid urbanization in APAC is driving the under floor-heating market as more people migrate to urban areas. This trend leads to a surge in residential and commercial construction projects, where modern heating solutions are increasingly preferred. Urban dwellers often seek efficient heating systems that can be seamlessly integrated into contemporary designs. The under floor-heating market benefits from this demand, as it offers a space-saving solution that enhances comfort. According to recent data, urban areas in APAC are expected to grow by approximately 50% by 2030, further propelling the need for innovative heating solutions. As cities expand, the adoption of under floor heating systems is likely to rise, making it a key driver in the market.
Rising Disposable Income
The increasing disposable income among consumers in APAC is contributing to the growth of the under floor-heating market. As households experience higher income levels, there is a noticeable shift towards investing in home improvement and comfort-enhancing solutions. Consumers are more inclined to opt for advanced heating systems that offer long-term savings on energy bills. Market data indicates that the average disposable income in APAC has risen by approximately 30% over the past decade, leading to a greater willingness to invest in quality heating solutions. This trend is expected to continue, further driving the demand for under floor heating systems as consumers prioritize comfort and efficiency in their homes.
Growing Awareness of Health Benefits
There is a growing awareness of the health benefits associated with under floor heating systems, which is positively influencing the market in APAC. Consumers are increasingly recognizing that under floor heating can reduce allergens and improve indoor air quality by eliminating the circulation of dust and other particles commonly associated with traditional heating methods. This awareness is particularly relevant in urban areas where air quality can be a concern. As health-conscious consumers seek solutions that promote well-being, the under floor-heating market is likely to see a rise in demand. Industry expert's suggests that this trend could lead to a 15% increase in sales of under floor heating systems over the next five years.
Government Initiatives for Energy Efficiency
Governments across APAC are implementing various initiatives aimed at promoting energy efficiency, which significantly impacts the under floor-heating market. Policies that encourage the adoption of energy-efficient technologies are becoming more prevalent, with incentives such as tax rebates and subsidies for homeowners and builders. For instance, several countries in the region have set ambitious targets to reduce energy consumption by 20% by 2030. These initiatives not only foster a favorable environment for the under floor-heating market but also raise awareness among consumers about the benefits of energy-efficient heating solutions. As a result, the market is likely to witness increased investments and innovations in under floor heating technologies.
Technological Advancements in Heating Solutions
Technological advancements are playing a crucial role in shaping the under floor-heating market in APAC. Innovations such as smart thermostats, energy-efficient heating cables, and advanced insulation materials are enhancing the performance and appeal of under floor heating systems. These technologies not only improve energy efficiency but also provide users with greater control over their heating preferences. The integration of smart home technologies is particularly noteworthy, as it allows for remote management of heating systems, thereby increasing convenience. As these advancements continue to evolve, they are likely to attract more consumers to the under floor-heating market, driving growth and adoption.