Rising Prevalence of Epilepsy
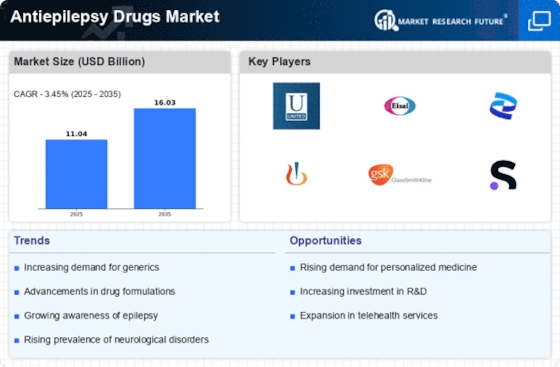
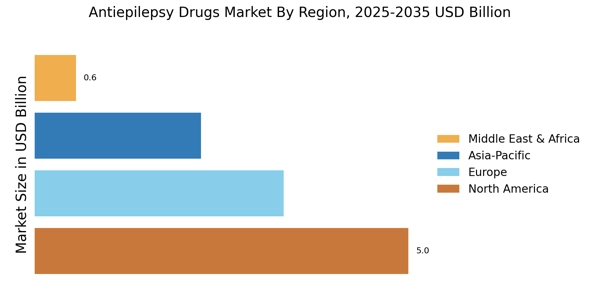
The increasing prevalence of epilepsy is a primary driver for the Antiepilepsy Drugs Market. According to estimates, approximately 50 million people worldwide are affected by epilepsy, with a notable rise in cases reported in various regions. This growing patient population necessitates the development and availability of effective antiepileptic medications. As awareness about epilepsy improves, more individuals are being diagnosed, which further fuels the demand for antiepilepsy drugs. The market is projected to expand as healthcare systems strive to provide adequate treatment options for this chronic neurological disorder. Consequently, pharmaceutical companies are likely to invest in research and development to introduce innovative therapies, thereby enhancing their market presence in the Antiepilepsy Drugs Market.
Growing Awareness and Education
There is a notable increase in awareness and education regarding epilepsy, which serves as a catalyst for the Antiepilepsy Drugs Market. Initiatives by healthcare organizations and advocacy groups aim to educate the public about epilepsy, its symptoms, and the importance of treatment. This heightened awareness leads to earlier diagnosis and treatment, thereby increasing the demand for antiepileptic drugs. Moreover, educational campaigns are encouraging patients to seek medical advice, which further propels the market growth. As more individuals become informed about their condition, the likelihood of them pursuing effective treatment options rises, thereby positively impacting the Antiepilepsy Drugs Market.
Advancements in Drug Development
Technological advancements in drug development are significantly influencing the Antiepilepsy Drugs Market. The introduction of novel drug delivery systems and formulations has improved the efficacy and safety profiles of antiepileptic medications. For instance, the development of long-acting formulations allows for less frequent dosing, which can enhance patient compliance. Furthermore, the integration of artificial intelligence in drug discovery processes is expediting the identification of potential therapeutic candidates. As a result, the market is witnessing a surge in the introduction of new drugs, which is expected to cater to the diverse needs of patients with epilepsy. This trend not only enhances treatment options but also drives competition among pharmaceutical companies within the Antiepilepsy Drugs Market.
Increase in Healthcare Expenditure
The rise in healthcare expenditure across various regions is contributing to the growth of the Antiepilepsy Drugs Market. Governments and private sectors are allocating more resources towards healthcare, which includes funding for neurological disorders such as epilepsy. This increase in expenditure facilitates better access to medications and treatment options for patients. Additionally, as healthcare systems evolve, there is a growing emphasis on providing comprehensive care for chronic conditions, including epilepsy. This trend is likely to result in higher demand for antiepileptic drugs, as patients seek effective management of their condition. Consequently, the Antiepilepsy Drugs Market is expected to benefit from this upward trajectory in healthcare investment.
Regulatory Support for Drug Approvals
Regulatory bodies are increasingly providing support for the approval of new antiepileptic drugs, which is a significant driver for the Antiepilepsy Drugs Market. Streamlined approval processes and incentives for the development of innovative therapies are encouraging pharmaceutical companies to invest in research and development. For instance, fast-track designations and priority review vouchers are being utilized to expedite the availability of new treatments. This regulatory environment fosters innovation and allows for a quicker response to the needs of patients suffering from epilepsy. As a result, the market is likely to see a rise in the number of approved antiepileptic drugs, enhancing treatment options available to patients within the Antiepilepsy Drugs Market.