Market Growth Projections
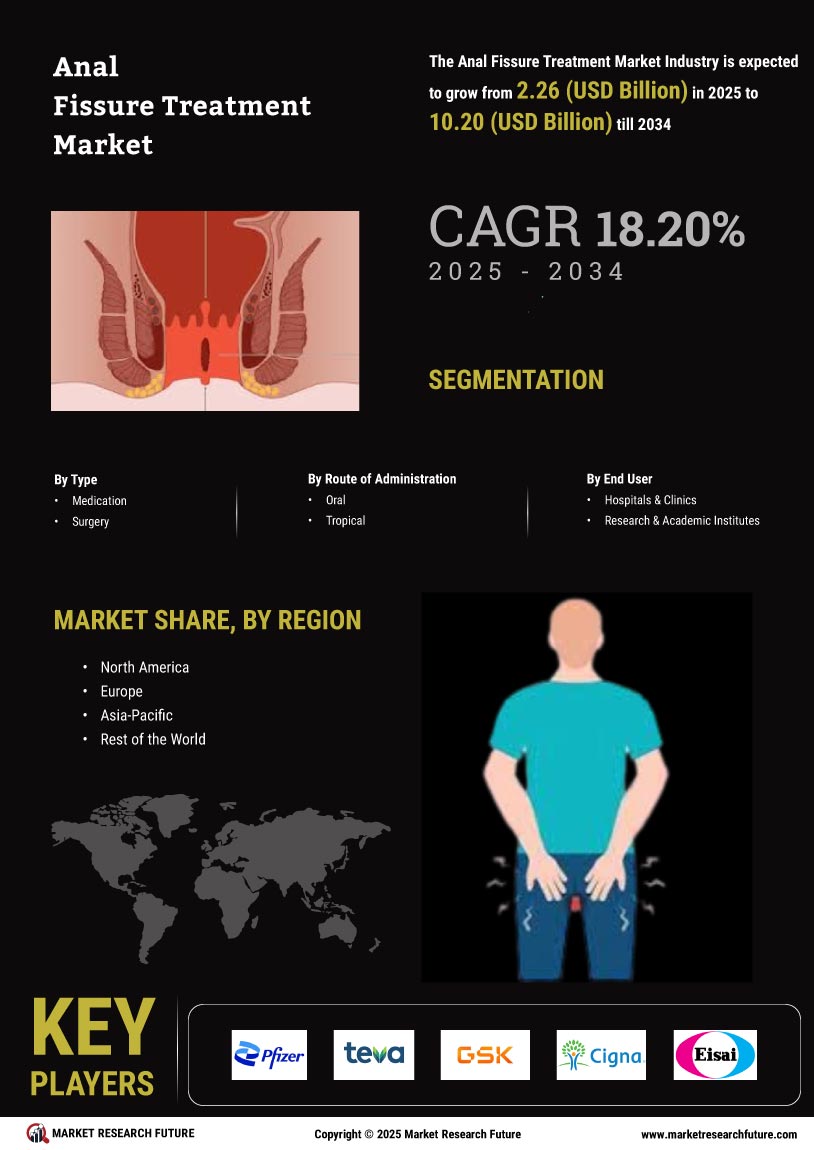
The Global Anal Fissure Treatment Market Industry is poised for substantial growth, with projections indicating a market value of 1.91 USD Billion in 2024 and an anticipated increase to 12.1 USD Billion by 2035. This growth trajectory reflects a compound annual growth rate (CAGR) of 18.23% from 2025 to 2035. Such figures underscore the potential for investment and innovation within the sector, as stakeholders recognize the expanding demand for effective anal fissure treatments. The market's evolution is likely to be shaped by ongoing research, technological advancements, and changing patient demographics.
Growing Geriatric Population
The aging population is a crucial factor impacting the Global Anal Fissure Treatment Market Industry. As individuals age, they may experience a higher incidence of anal fissures due to factors such as decreased elasticity of anal tissues and comorbidities that affect bowel health. The World Health Organization projects that the global population aged 60 years and older will reach 2.1 billion by 2050. This demographic shift is likely to result in increased demand for anal fissure treatments, thereby driving market growth. Healthcare systems must adapt to meet the needs of this population, emphasizing the importance of effective treatment options.
Rising Healthcare Expenditure
The escalation of healthcare expenditure globally is positively influencing the Global Anal Fissure Treatment Market Industry. As countries invest more in healthcare infrastructure and services, access to treatment for anal fissures improves. Increased funding for research and development leads to the introduction of innovative therapies and better patient care. For instance, nations with robust healthcare systems are more likely to provide comprehensive treatment options, enhancing patient outcomes. This trend is expected to contribute to the market's growth, as higher healthcare spending correlates with increased utilization of anal fissure treatments.
Increased Awareness and Education
Heightened awareness regarding anal fissures and their treatment options is driving the Global Anal Fissure Treatment Market Industry. Public health campaigns and educational initiatives by healthcare organizations aim to destigmatize discussions surrounding anal health, encouraging individuals to seek timely medical intervention. This increased awareness is likely to lead to higher diagnosis rates and, consequently, a greater demand for treatment. As more patients become informed about available therapies, the market is expected to expand, with significant implications for healthcare providers and pharmaceutical companies alike.
Rising Prevalence of Anal Disorders
The increasing incidence of anal disorders, including anal fissures, is a primary driver of the Global Anal Fissure Treatment Market Industry. Factors such as dietary habits, sedentary lifestyles, and an aging population contribute to this rise. For instance, studies indicate that approximately 1 in 10 individuals may experience anal fissures at some point in their lives. This growing prevalence is expected to propel the market, with projections estimating the market value to reach 1.91 USD Billion in 2024 and potentially surge to 12.1 USD Billion by 2035, reflecting a compound annual growth rate (CAGR) of 18.23% from 2025 to 2035.
Advancements in Treatment Modalities
Innovations in treatment options for anal fissures are significantly influencing the Global Anal Fissure Treatment Market Industry. The introduction of minimally invasive procedures, such as lateral internal sphincterotomy and the use of topical nitroglycerin, has improved patient outcomes and reduced recovery times. Furthermore, the development of new pharmacological agents, including calcium channel blockers and botulinum toxin injections, offers alternative therapies that may enhance healing. These advancements not only improve patient satisfaction but also contribute to the market's growth trajectory, as healthcare providers increasingly adopt these effective treatment modalities.