Increased Focus on Rare Diseases
The growing emphasis on rare diseases, including Alagille Syndrome Market, is shaping the landscape of the Alagille Syndrome Market. Policymakers and healthcare systems are increasingly prioritizing the development of treatments for rare conditions, driven by the recognition of their unique challenges. This focus is reflected in the establishment of incentives for pharmaceutical companies to invest in research and development for rare diseases. As a result, the Alagille Syndrome Market may benefit from increased funding and resources, facilitating the discovery of new therapies and improving access to existing treatments. This trend indicates a broader commitment to addressing the needs of patients with rare genetic disorders.
Advancements in Treatment Options
Innovations in treatment options for Alagille Syndrome Market are significantly influencing the Alagille Syndrome Market. Recent developments in targeted therapies and gene editing technologies hold promise for improving patient outcomes. For instance, the introduction of novel medications aimed at managing cholestasis and other symptoms associated with the syndrome has the potential to enhance the quality of life for affected individuals. Furthermore, clinical trials are increasingly exploring the efficacy of these new treatments, which may lead to their approval and subsequent market entry. As a result, the Alagille Syndrome Market is likely to experience a surge in investment and interest from both pharmaceutical companies and healthcare providers, eager to capitalize on these advancements.
Growing Support from Advocacy Groups
The role of advocacy groups in raising awareness and supporting research for Alagille Syndrome Market is a crucial driver in the Alagille Syndrome Market. Organizations dedicated to this condition are actively working to educate the public and healthcare professionals about the syndrome, thereby increasing the rate of diagnosis and treatment. These groups often collaborate with researchers and pharmaceutical companies to fund studies and clinical trials, which can lead to the development of new therapies. The heightened visibility and support from these organizations may encourage more investment in the Alagille Syndrome Market, as stakeholders recognize the potential for growth and innovation in addressing the needs of patients.
Rising Prevalence of Alagille Syndrome
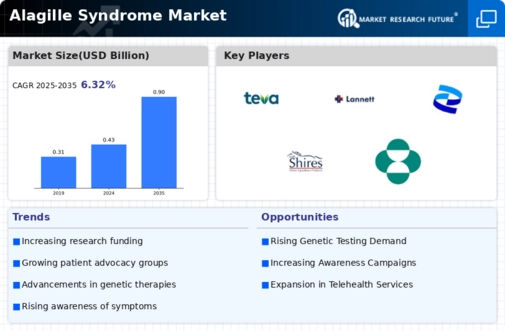
The increasing prevalence of Alagille Syndrome Market is a notable driver in the Alagille Syndrome Market. Recent estimates suggest that the incidence of this genetic disorder ranges from 1 in 30,000 to 1 in 50,000 live births. This rise in diagnosed cases is likely to lead to a greater demand for specialized treatments and healthcare services. As more individuals are identified with this condition, healthcare providers are compelled to enhance their focus on tailored therapies and management strategies. Consequently, this trend may stimulate growth in the Alagille Syndrome Market, as pharmaceutical companies and research institutions invest in developing innovative solutions to address the unique challenges posed by this syndrome.
Technological Innovations in Diagnostics
Technological advancements in diagnostic tools are transforming the Alagille Syndrome Market. Enhanced genetic testing methods, such as next-generation sequencing, are enabling earlier and more accurate diagnoses of Alagille Syndrome Market. This shift towards precision medicine allows for timely intervention and management of the condition, which is crucial for improving patient outcomes. As diagnostic capabilities improve, healthcare providers are likely to identify more cases, leading to an increased demand for treatment options. Consequently, the Alagille Syndrome Market is poised for growth as stakeholders respond to the rising need for effective management strategies and therapies tailored to this complex disorder.