Point Of Care Diagnostics Testing Size
Point of Care Diagnostics Testing Market Growth Projections and Opportunities
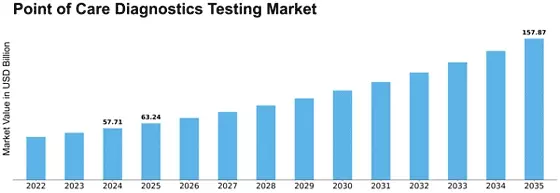
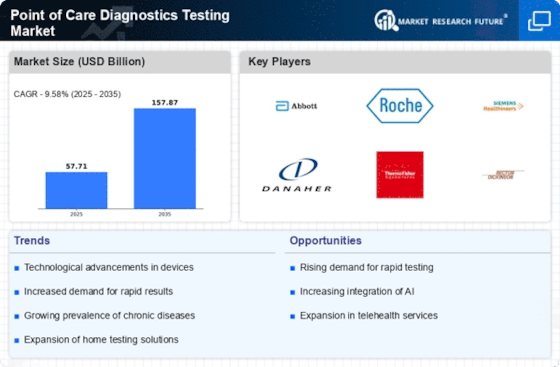
The size of the market for point-of-care diagnostics/testing is at a CAGR of 11.5% with USD 19.12 Billion over the forecast period from 2022 to 2030. The market is driven by several factors that together define its outline and growth trajectory. One such major force driving this paradigm shift is an increased focus on personalized and patient-centric healthcare service delivery models. Point-of-care-diagnostic testing, as a rapid and decentralized test, goes along with the changing norm of providing accurate information when needed by patients. Technological advances are important influencers in shaping the POC diagnostic market. Innovations in diagnostic technologies like biosensors, molecular diagnostics, and portable imaging devices contribute to the development of faster and more accurate point-of-care test tools. Diagnostic devices are being made smaller while lab-on-a-chip technologies have been employed, leading to tests being carried out outside typical laboratories. Epidemiological factors also influence the demand for points-of-care diagnostics solutions. Infectious disease prevalence; increasing burden of chronic conditions like diabetes, hypertension, etc.; rapid screening required in emergency/urgent care settings drive adoption of POC testing solutions. Point Of Care Diagnostics Market depends on regulatory frameworks, and approval processes are key influences. Stringent adherence to regulatory standards ensures the safety & efficacy of such devices in POCT applications; Regulatory approvals must be secured for entry into the place requiring complex process navigation for companies before commercialization can take place through their products; Getting permissions from national regulators around the world has never been easy. The adoption rate for point-of-care diagnostics is significantly influenced by economic factors such as cost-effectiveness and affordability. Global collaboration on health infrastructure will support expansion in point-of-care diagnostics markets. It would be easy to integrate PoC diagnostics globally if governments collaborated with international organizations as well as industry players themselves. Market dynamics for the Point Of Care Diagnostics Market include competition and consolidation among companies. The landscape of this field is characterized by major players, the entry of new firms, and strategic alliances, among other factors, for market participants such as manufacturers; product portfolio expansion and company strengthening are done through acquisitions, mergers, and research partnerships.

















Leave a Comment