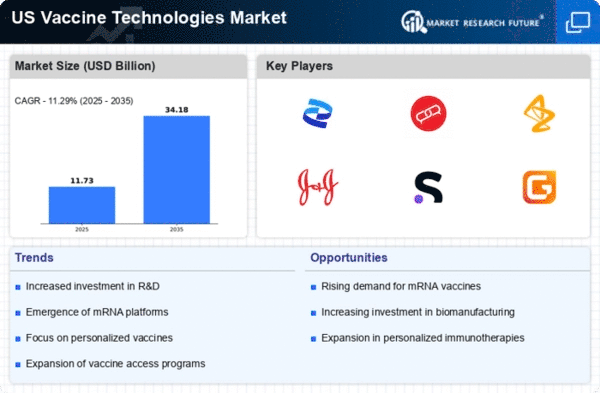
Government Initiatives and Funding
Government initiatives and funding play a pivotal role in shaping the vaccine technologies market. In the US, federal and state governments are increasingly investing in vaccine research and development to bolster public health infrastructure. Programs aimed at enhancing vaccine accessibility and affordability are being implemented, which may lead to a more robust market environment. For instance, the US government allocated over $10 billion in funding for vaccine development initiatives in recent years. Such financial support not only stimulates innovation but also encourages collaboration between public and private sectors, thereby fostering growth within the vaccine technologies market.
Rising Demand for Preventive Healthcare
The increasing emphasis on preventive healthcare is a notable driver for the vaccine technologies market. As healthcare systems in the US shift towards proactive measures, the demand for vaccines is expected to rise. This trend is reflected in the growing public awareness regarding the importance of vaccinations in preventing diseases. According to recent data, the market for vaccines is projected to reach approximately $60 billion by 2026, indicating a compound annual growth rate (CAGR) of around 8%. This shift towards preventive healthcare not only enhances public health outcomes but also drives innovation within the vaccine technologies market, as companies strive to develop more effective and accessible vaccines.
Technological Innovations in Vaccine Production
Technological advancements in vaccine production are significantly influencing the vaccine technologies market. Innovations such as recombinant DNA technology and viral vector platforms are enhancing the efficiency and efficacy of vaccine development. These technologies allow for faster production times and improved safety profiles, which are crucial in responding to emerging health threats. The market is witnessing a surge in investments, with estimates suggesting that the vaccine production technology segment could grow to $25 billion by 2025. This growth is indicative of the industry's commitment to leveraging cutting-edge technologies to meet the evolving needs of public health.
Increased Focus on Infectious Disease Preparedness
The heightened focus on infectious disease preparedness is a critical driver for the vaccine technologies market. The US has recognized the necessity of being better equipped to handle potential outbreaks, leading to increased investments in vaccine research and development. This proactive approach is reflected in the establishment of various public-private partnerships aimed at accelerating vaccine innovation. The market is expected to benefit from this focus, with projections indicating a potential growth rate of 7% annually through 2030. This emphasis on preparedness not only enhances the vaccine technologies market but also strengthens the overall healthcare system.
Growing Public Awareness and Acceptance of Vaccines
The growing public awareness and acceptance of vaccines are vital factors influencing the vaccine technologies market. Educational campaigns and outreach programs have significantly improved public perception regarding the safety and efficacy of vaccines. This shift in attitude is crucial, as it directly impacts vaccination rates and, consequently, market growth. Recent surveys indicate that approximately 80% of the US population supports vaccination initiatives, which is likely to drive demand for new vaccine technologies. As acceptance continues to rise, the vaccine technologies market is poised for expansion, with companies focusing on developing innovative solutions to meet this increasing demand.