Growing Investment in R&D
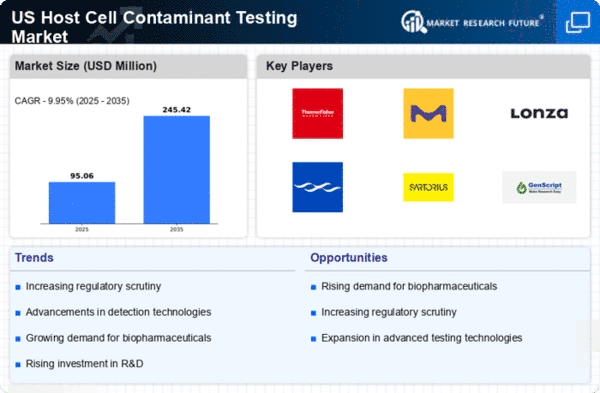
The increasing investment in research and development (R&D) within the biopharmaceutical sector is a significant driver for the host cell-contaminant-testing market. As companies strive to develop new therapies and improve existing products, the need for comprehensive testing becomes paramount. In 2025, R&D spending in the biopharmaceutical industry is anticipated to exceed $100 billion in the US, underscoring the importance of robust testing frameworks. This investment not only supports the development of innovative therapies but also necessitates the implementation of stringent testing protocols to ensure safety and efficacy. Consequently, the host cell-contaminant-testing market is poised for growth as R&D activities expand.
Rising Demand for Biologics
The increasing demand for biologics in the pharmaceutical sector is a primary driver for the host cell-contaminant-testing market. As biologics become more prevalent in treating various diseases, the need for rigorous testing to ensure product safety and efficacy intensifies. In 2025, the biologics market is projected to reach approximately $500 billion in the US, highlighting the necessity for effective contaminant testing. This surge in biologics production necessitates stringent testing protocols to mitigate risks associated with host cell contaminants, thereby propelling the growth of the host cell-contaminant-testing market. Companies are investing in advanced testing technologies to meet regulatory requirements and consumer expectations, which further stimulates market expansion.
Increased Regulatory Scrutiny
Regulatory bodies in the US are imposing stricter guidelines regarding the safety and quality of biopharmaceutical products, which significantly impacts the host cell-contaminant-testing market. The FDA and other regulatory agencies are emphasizing the importance of thorough testing to prevent contamination that could compromise patient safety. As a result, pharmaceutical companies are compelled to adopt comprehensive testing strategies to comply with these regulations. the market for host cell-contaminant testing was expected to grow at a CAGR of around 10% through 2025, driven by the need for compliance with these evolving regulatory standards. This heightened scrutiny not only ensures product integrity but also fosters consumer trust in biopharmaceuticals.
Technological Innovations in Testing
Technological advancements in testing methodologies are transforming the host cell-contaminant-testing market. Innovations such as next-generation sequencing and advanced chromatography techniques are enhancing the sensitivity and specificity of contaminant detection. These technologies enable faster and more accurate testing, which is crucial for biopharmaceutical companies aiming to streamline their production processes. The integration of automation and artificial intelligence in testing protocols is also gaining traction, potentially reducing operational costs and improving efficiency. As the market evolves, companies that leverage these technological innovations are likely to gain a competitive edge, thereby driving growth in the host cell-contaminant-testing market.
Rising Consumer Awareness and Safety Concerns
There is a notable increase in consumer awareness regarding the safety and quality of biopharmaceutical products, which is influencing the host cell-contaminant-testing market. Patients and healthcare providers are becoming more informed about the potential risks associated with contaminants in biologics. This heightened awareness is driving demand for rigorous testing to ensure that products are free from harmful contaminants. As a result, pharmaceutical companies are prioritizing safety measures and investing in comprehensive testing solutions. The host cell-contaminant-testing market is likely to benefit from this trend, as companies seek to enhance their testing capabilities to meet consumer expectations and regulatory requirements.