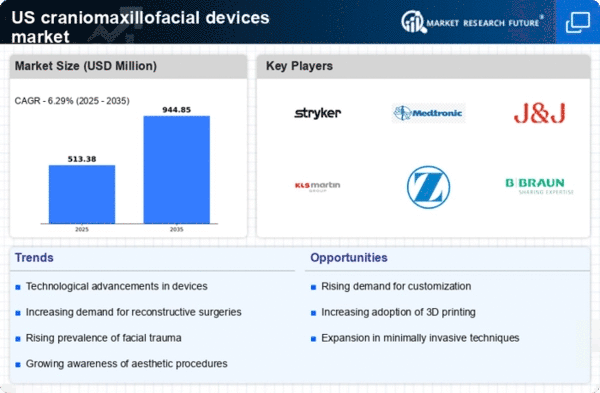
Supportive Regulatory Environment
A supportive regulatory environment is a critical driver for the craniomaxillofacial devices market. The US Food and Drug Administration (FDA) has established streamlined pathways for the approval of innovative medical devices, facilitating quicker access to the market. This regulatory support encourages manufacturers to invest in research and development, leading to the introduction of advanced craniomaxillofacial devices. Furthermore, the FDA's emphasis on safety and efficacy ensures that high-quality products are available to healthcare providers and patients. The market is also bolstered by initiatives aimed at reducing regulatory burdens, which can expedite the approval process for new technologies. As a result, the favorable regulatory landscape is likely to foster innovation and growth within the craniomaxillofacial devices market.
Increasing Incidence of Facial Trauma
The rising incidence of facial trauma in the US is a notable driver for the craniomaxillofacial devices market. Factors such as road accidents, sports injuries, and interpersonal violence contribute to this trend. According to the National Highway Traffic Safety Administration, there were over 38,000 fatalities in motor vehicle crashes in 2022, many resulting in facial injuries. This growing prevalence necessitates advanced surgical interventions and devices, thereby propelling market growth. Furthermore, the American Association of Oral and Maxillofacial Surgeons indicates that approximately 1.5 million patients undergo facial reconstructive surgery annually, highlighting the demand for innovative craniomaxillofacial devices. As the population continues to engage in high-risk activities, the need for effective treatment solutions will likely increase, further stimulating the market.
Rising Awareness of Aesthetic Procedures
The growing awareness and acceptance of aesthetic procedures among the US population is a notable driver for the craniomaxillofacial devices market. As societal norms shift towards valuing physical appearance, more individuals are seeking surgical options to enhance their facial features. The American Society of Plastic Surgeons reported that nearly 18 million cosmetic procedures were performed in 2022, with a significant portion involving facial enhancements. This trend is expected to continue, as younger demographics increasingly prioritize aesthetic improvements. Additionally, the rise of social media platforms has amplified the desire for aesthetic perfection, further fueling demand for craniomaxillofacial devices. As more individuals opt for cosmetic surgeries, the market is likely to experience substantial growth, driven by the need for innovative and effective solutions.
Aging Population and Associated Disorders
The aging population in the US is a significant driver for the craniomaxillofacial devices market. As individuals age, they become more susceptible to various health issues, including bone density loss and facial deformities. The US Census Bureau projects that by 2030, nearly 20% of the population will be 65 years or older, leading to a higher demand for surgical interventions. Conditions such as osteoporosis and other degenerative diseases often necessitate the use of craniomaxillofacial devices for reconstruction and repair. Additionally, the American Geriatrics Society reports that older adults are more likely to experience facial fractures, further increasing the need for specialized devices. This demographic shift suggests a sustained growth trajectory for the market as healthcare providers seek to address the unique needs of an aging population.
Technological Innovations in Surgical Procedures
Technological innovations play a crucial role in driving the craniomaxillofacial devices market. Advancements in 3D printing, imaging technologies, and biomaterials have revolutionized surgical procedures, enhancing precision and outcomes. For instance, 3D-printed implants are increasingly utilized for their customization capabilities, allowing for tailored solutions that improve patient satisfaction. The market for 3D-printed medical devices is projected to reach $6 billion by 2026, indicating a robust growth potential. Moreover, the integration of augmented reality in surgical planning is gaining traction, enabling surgeons to visualize complex anatomical structures more effectively. These innovations not only improve surgical efficiency but also reduce recovery times, thereby driving demand for craniomaxillofacial devices. As technology continues to evolve, it is likely to further transform the landscape of surgical interventions.