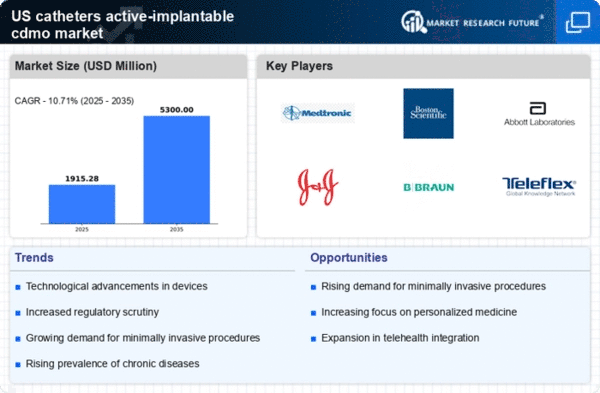
Rising Prevalence of Chronic Diseases
The increasing incidence of chronic diseases such as cardiovascular disorders, diabetes, and renal failure is a primary driver for the catheters active-implantable-cdmo market. As these conditions often necessitate the use of catheters for treatment and management, the demand for innovative catheter solutions is likely to rise. According to recent data, chronic diseases affect nearly 60% of the adult population in the US, leading to a substantial market opportunity. The need for effective management of these diseases through catheterization is expected to propel the growth of the market, as healthcare providers seek advanced solutions to improve patient outcomes. This trend indicates a robust demand for catheters that are not only effective but also designed for long-term use, thereby enhancing the overall market landscape.
Growing Demand for Home Healthcare Solutions
The shift towards home healthcare solutions is emerging as a significant driver for the catheters active-implantable-cdmo market. As patients increasingly prefer receiving care in the comfort of their homes, the demand for portable and user-friendly catheter systems is likely to grow. This trend is supported by the aging population and the rising prevalence of chronic conditions that require ongoing management. It is estimated that the home healthcare market will expand at a CAGR of 10% over the next few years, creating opportunities for catheter manufacturers to develop products tailored for home use. This shift not only enhances patient convenience but also reduces healthcare costs, thereby influencing the overall market dynamics.
Technological Innovations in Catheter Design
Technological advancements in catheter design and materials are significantly influencing the catheters active-implantable-cdmo market. Innovations such as biocompatible materials, smart catheters with integrated sensors, and enhanced delivery systems are becoming increasingly prevalent. These advancements not only improve the functionality and safety of catheters but also cater to the growing demand for personalized medicine. The market is projected to witness a growth rate of approximately 8% annually, driven by these innovations. As manufacturers invest in research and development to create next-generation catheters, the market is likely to expand, offering healthcare providers more effective tools for patient care. This focus on innovation is essential for maintaining competitiveness in the evolving landscape of medical devices.
Increased Investment in Healthcare Infrastructure
The ongoing investment in healthcare infrastructure in the US is a crucial driver for the catheters active-implantable-cdmo market. With the expansion of hospitals, outpatient facilities, and specialized clinics, the demand for advanced medical devices, including catheters, is expected to rise. Recent reports indicate that healthcare spending in the US is projected to reach $4 trillion by 2025, reflecting a growing commitment to improving patient care. This investment not only enhances the availability of medical services but also encourages the adoption of innovative catheter technologies. As healthcare facilities upgrade their equipment and services, the catheters active-implantable-cdmo market stands to benefit from increased procurement of advanced catheter solutions.
Regulatory Support for Innovative Medical Devices
Regulatory support for the development and approval of innovative medical devices is a vital driver for the catheters active-implantable-cdmo market. The US Food and Drug Administration (FDA) has implemented streamlined processes for the approval of advanced catheter technologies, facilitating quicker market entry for new products. This regulatory environment encourages manufacturers to invest in research and development, fostering innovation within the industry. As a result, the market is likely to see a surge in the introduction of novel catheter solutions that meet evolving healthcare needs. The proactive stance of regulatory bodies in supporting innovation is expected to enhance the competitive landscape of the catheters active-implantable-cdmo market.