Advancements in Treatment Modalities
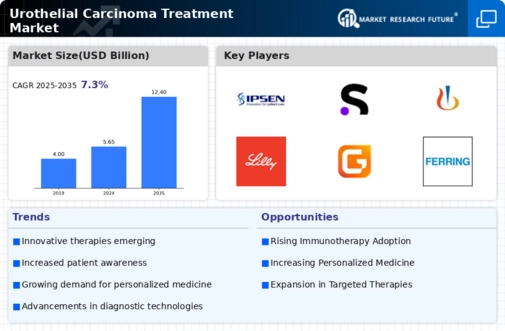
Recent advancements in treatment modalities are significantly influencing the Urothelial Carcinoma Treatment Market. The introduction of novel therapies, including immune checkpoint inhibitors and targeted therapies, has transformed the treatment landscape. For instance, drugs such as pembrolizumab and atezolizumab have shown promising results in clinical trials, leading to their approval for use in advanced urothelial carcinoma. These innovations not only improve patient outcomes but also expand the therapeutic options available to oncologists. The ongoing research and development efforts in this area suggest a potential for further breakthroughs, which could enhance the efficacy of treatments and drive market growth in the Urothelial Carcinoma Treatment Market.
Growing Investment in Cancer Research
The increasing investment in cancer research is a crucial driver for the Urothelial Carcinoma Treatment Market. Governments and private organizations are allocating substantial funds to support research initiatives aimed at understanding the biology of urothelial carcinoma and developing new treatment strategies. For example, funding for clinical trials has seen a notable rise, with millions of dollars directed towards exploring innovative therapies. This financial commitment not only accelerates the pace of research but also fosters collaboration between academic institutions and pharmaceutical companies. As a result, the Urothelial Carcinoma Treatment Market is likely to benefit from a pipeline of new therapies that could emerge from these research endeavors.
Rising Awareness and Screening Programs
The growing awareness of urothelial carcinoma and the implementation of screening programs are pivotal factors driving the Urothelial Carcinoma Treatment Market. Educational campaigns aimed at both healthcare professionals and the general public have increased knowledge about the risk factors and symptoms associated with bladder cancer. Consequently, more individuals are seeking medical attention, leading to earlier diagnosis and treatment. Additionally, various organizations are promoting routine screening for high-risk populations, which may further enhance early detection rates. This heightened awareness not only contributes to improved patient outcomes but also stimulates demand for treatment options, thereby positively impacting the Urothelial Carcinoma Treatment Market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a significant driver in the Urothelial Carcinoma Treatment Market. Regulatory agencies are increasingly adopting expedited review processes for new cancer treatments, recognizing the urgent need for effective therapies in oncology. This trend is exemplified by the accelerated approval pathways established for breakthrough therapies, which allow for faster access to promising treatments for patients. Such regulatory frameworks encourage pharmaceutical companies to invest in the development of novel therapies for urothelial carcinoma. As a result, the Urothelial Carcinoma Treatment Market is likely to witness a surge in new product launches, enhancing the overall treatment landscape.
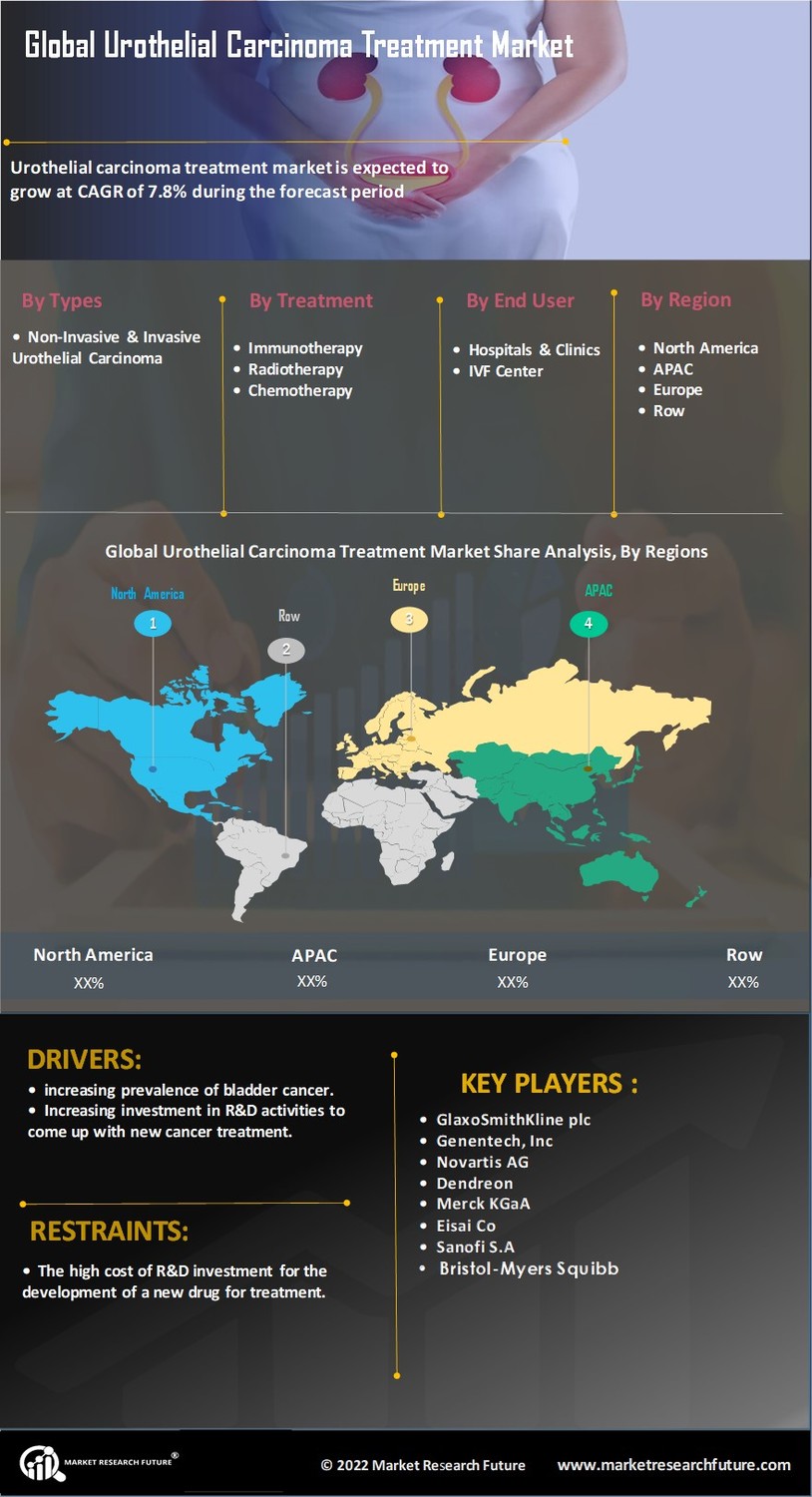
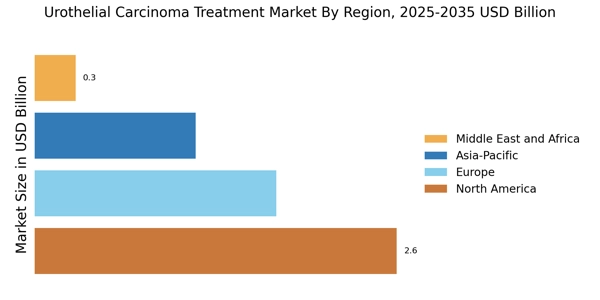
Increasing Incidence of Urothelial Carcinoma
The rising incidence of urothelial carcinoma is a primary driver for the Urothelial Carcinoma Treatment Market. According to recent data, the prevalence of bladder cancer, which includes urothelial carcinoma, has been steadily increasing, with an estimated 81,000 new cases reported annually. This surge in cases necessitates the development and availability of effective treatment options, thereby propelling market growth. Furthermore, the aging population is likely to contribute to this trend, as older individuals are at a higher risk for developing this type of cancer. As awareness of the disease grows, healthcare providers are increasingly focused on early detection and intervention, which may further stimulate demand for innovative therapies within the Urothelial Carcinoma Treatment Market.