Research Methodology on Tumor Ablation Market
1. Introduction
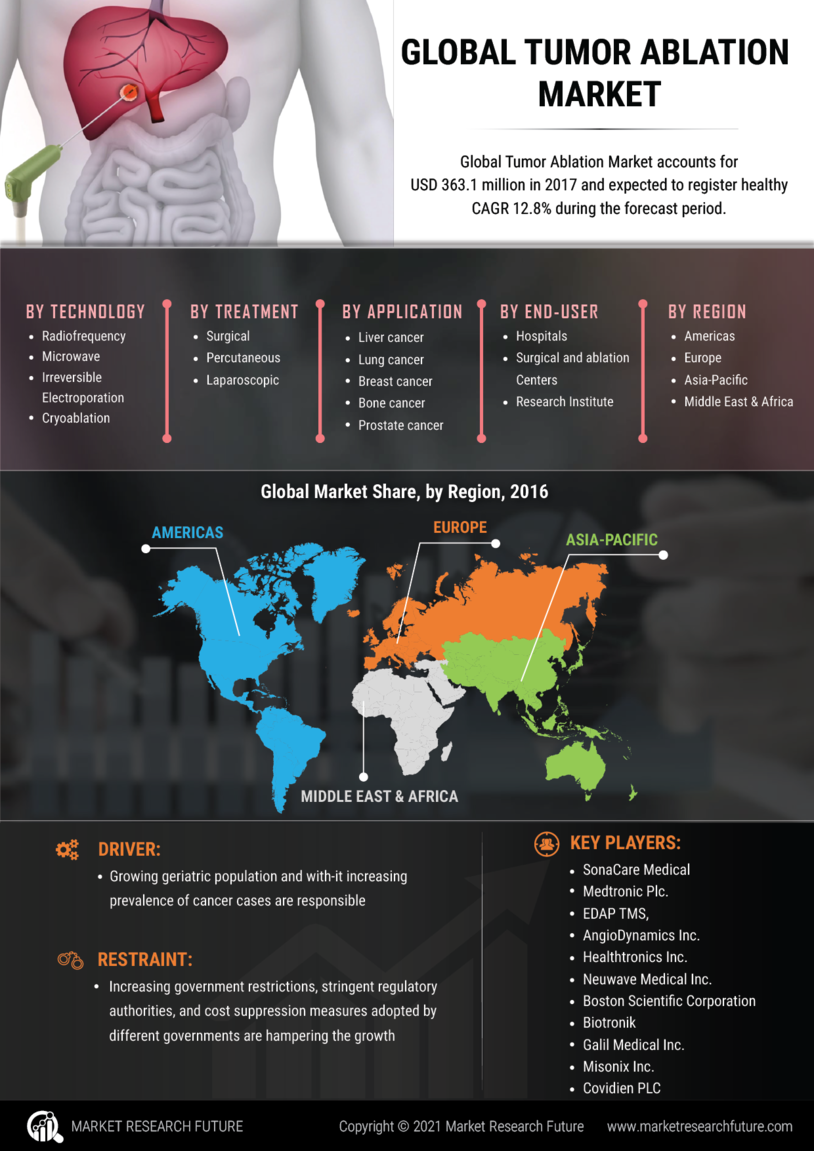
The tumor ablation market is a global source for research and development activities related to the use of ablation therapy for tumors such as the liver, lung, kidney, and prostate. It is segmented into both internal and external ablation techniques, product and service-related developments, research trials, case studies, and regional market analysis to provide information about the device types, ablation techniques, therapeutic outcomes, and safety profiles. This research provides an overview of the current market trends and estimates associated with tumor ablation technology and provides both qualitative and quantitative insights into the market. The study focuses on the key market dynamics, industry size, revenue, and geographical trends.
2. Research Objectives and Goals
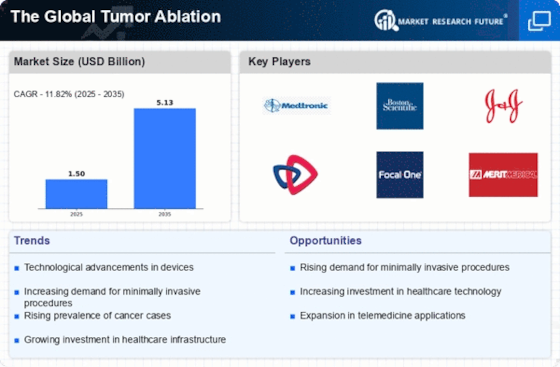
The primary goal of this research is to provide a comprehensive overview of the current state of the tumor ablation market. In particular, the research seeks to provide a market size, segmentation, and scenario analysis and identify potential opportunities for market expansion in the future. In addition, the research identifies the current and future trends in the tumor ablation market and the associated challenges to global market participants. The scope of the research includes insights into the market overview, technology and product ideation, clinical and economic analysis, market adoption, clinical trial and regulatory developments, major players and their strategies, and global market opportunities and challenges.
3. Research methodology
This research is based on a comprehensive and extensive market research methodology, supplemented with secondary research, industry interviews and data analysis to obtain detailed insights into the tumor ablation market. For this purpose, we have adopted both primary and secondary research sources, including in-depth interviews with industry participants, customized surveys and macro-economic trends. Various secondary sources, such as white papers and market data, are used to provide a comprehensive view of the tumor ablation market. The analysis is supplemented with a thorough assessment of the economic, socio-economic, political and technological aspects of the global tumor ablation market. Additionally, in-depth interviews with industry participants have been conducted to gain insights into their opinion and views on the current and future scenarios for the market.
4. Research Framework
The research framework consists of the following steps:
- Market Overview: In the market overview step, the research team will provide an overview of the market, including its current state and development, with a focus on drivers, restraints and opportunities associated with the growing adoption of tumor ablation technology globally.
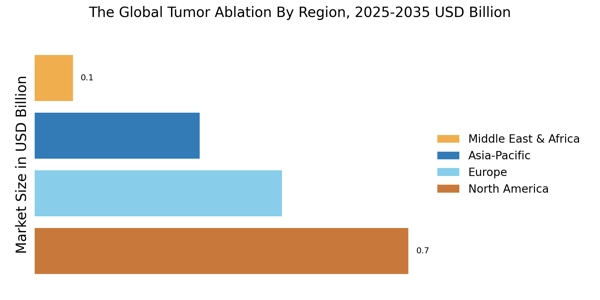
- Market Segmentation: The segmentation step involves the identification and classification of different segments related to the tumor ablation market, such as technology, product type, patient population, therapeutic application, and regional demand.
- Market Dynamics: This step includes the identification and analysis of the current and emerging trends associated with the global tumor ablation market.
- Market Scenario: The market scenario step involves a thorough assessment of the overall market dynamics, including insights into the competitive landscape, patent analysis, and regulatory framework.
- Research & Development: The research & development step includes the identification and evaluation of recent product innovations and research developments related to the tumor ablation market.
- Business & Regulatory Environment: This step involves the identification and analysis of the key regulatory frameworks, current market trends and economic analysis of the tumor ablation market.
5. Data Collection Methodology
The research is based on both primary and secondary sources of research. For the primary research, interviews were conducted with key industry participants, such as manufacturers, distributors and end users, to obtain a comprehensive understanding of the current and future scenarios for the market. The industry interviews were conducted through telephonic interviews, online survey forms and in-person meetings. To obtain insights into the latest market trends and developments, the research team employed both primary and secondary research processes. Secondary sources, such as published reports and white papers, were extensively used to obtain a detailed understanding of the market.
6. Data Analysis Methodology
The research team used both quantitative and qualitative analyses to obtain a comprehensive understanding of the tumor ablation market. Both primary and secondary sources of data were used to quantify and analyze the data. Quantitative analyses include market scenario assessment, segmentation, market size estimation and market forecast using a combination of regression, qualitative and quantitative methods.
To supplement the primary and secondary sources, in-depth interviews were conducted with industry participants, such as manufacturers, distributors and end users. The research team also conducted a thorough market analysis to identify the competitive landscape, market dynamics and key industry trends.
7. Conclusion
This report is a comprehensive overview of the tumor ablation market, which details its current development and future prospects. Through data collection, analysis and market assessment, the report provides extensive insight into the evolution of the tumor ablation market and its key strategies and strategies. The report provides an extensive understanding of the current market dynamics, key players, technology advancements and opportunities for expansion for the global tumor ablation market.