Regulatory Framework Enhancements
The evolving regulatory framework in Spain is playing a pivotal role in shaping the singleplex immunoassay market. Recent updates to regulations governing diagnostic tests are aimed at ensuring safety and efficacy, which in turn boosts confidence among healthcare providers and patients. The singleplex immunoassay market is likely to see increased adoption as regulatory bodies streamline approval processes for new assays. This regulatory support not only facilitates market entry for innovative products but also encourages manufacturers to invest in the development of high-quality assays. Consequently, the alignment of regulatory standards with market needs is expected to drive growth in the singleplex immunoassay market.
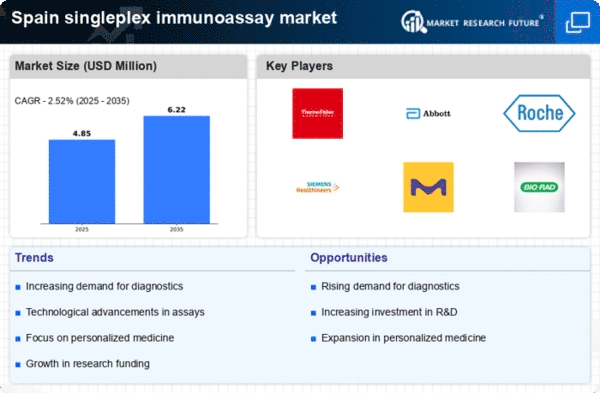
Rising Demand for Diagnostic Testing
The increasing prevalence of chronic diseases in Spain is driving the demand for diagnostic testing, particularly in the singleplex immunoassay market. As healthcare providers seek efficient and accurate testing methods, singleplex immunoassays offer a streamlined approach to detect specific biomarkers. The market is projected to grow at a CAGR of approximately 8% over the next five years, reflecting the urgent need for rapid diagnostics. This growth is further fueled by the aging population, which is more susceptible to various health conditions. Consequently, the singleplex immunoassay market is positioned to benefit from this rising demand, as healthcare systems prioritize early detection and management of diseases.
Growing Focus on Preventive Healthcare
The shift towards preventive healthcare in Spain is significantly influencing the singleplex immunoassay market. With an emphasis on early detection and disease prevention, healthcare providers are increasingly utilizing singleplex immunoassays to monitor health markers. This proactive approach is supported by public health campaigns aimed at educating the population about the importance of regular health screenings. The singleplex immunoassay market is likely to thrive as more individuals seek out these tests to manage their health proactively. Furthermore, the integration of these assays into routine check-ups could lead to a substantial increase in testing volumes, thereby enhancing market growth.
Advancements in Laboratory Infrastructure
Spain's investment in modernizing laboratory infrastructure is a crucial driver for the singleplex immunoassay market. Enhanced laboratory capabilities enable the adoption of advanced testing technologies, including singleplex immunoassays, which provide precise and reliable results. The Spanish government has allocated substantial funding to improve healthcare facilities, which is expected to increase the efficiency of diagnostic processes. As a result, the singleplex immunoassay market is likely to experience growth, with laboratories increasingly integrating these assays into their testing repertoire. This modernization not only improves patient outcomes but also streamlines laboratory workflows, making it a pivotal factor in the market's expansion.
Increased Research and Development Activities
The surge in research and development activities within Spain's biotechnology sector is a notable driver for the singleplex immunoassay market. Academic institutions and private companies are investing in innovative assay technologies, which are essential for advancing diagnostic capabilities. This focus on R&D is likely to yield new and improved singleplex immunoassays, enhancing their sensitivity and specificity. As a result, the singleplex immunoassay market is expected to benefit from the introduction of novel products that meet the evolving needs of healthcare providers. The collaboration between research entities and healthcare organizations further strengthens this trend, fostering an environment conducive to innovation.