Regulatory Evolution
The evolving regulatory landscape in Spain is playing a crucial role in shaping the preclinical cro market. Recent updates to regulatory frameworks are designed to streamline the approval process for new drugs, which may enhance the attractiveness of preclinical studies. In 2025, it is anticipated that regulatory agencies will implement more flexible guidelines, potentially reducing the time required for preclinical testing. This evolution is likely to encourage pharmaceutical companies to engage CROs for their preclinical needs, as they seek to navigate the complexities of compliance efficiently. The preclinical cro market stands to benefit from this regulatory evolution, as it may lead to increased demand for services that ensure adherence to new standards.
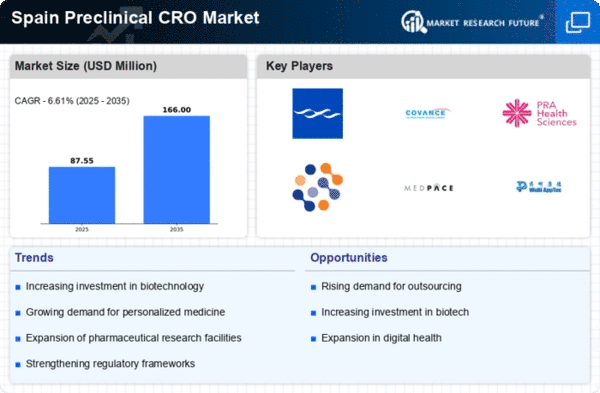
Investment in Biotechnology
Spain's preclinical cro market is benefiting from increased investment in biotechnology. The Spanish government has been actively promoting biotechnology initiatives, with funding reaching approximately €500 million in 2025. This financial support is likely to stimulate research activities and foster collaborations between academic institutions and CROs. As biotechnology firms seek to advance their product pipelines, the demand for preclinical services is expected to rise. The focus on innovative biopharmaceuticals, including monoclonal antibodies and gene therapies, necessitates robust preclinical testing to validate safety and efficacy. Consequently, the preclinical cro market is poised for growth as it aligns with the burgeoning biotechnology sector in Spain.
Focus on Personalized Medicine
The preclinical cro market is increasingly influenced by the growing emphasis on personalized medicine in Spain. As healthcare providers and researchers recognize the importance of tailoring treatments to individual patient profiles, the demand for preclinical studies that support this approach is likely to rise. In 2025, it is estimated that around 30% of new drug candidates will be developed with a focus on personalized therapies. This shift necessitates advanced preclinical testing to ensure efficacy and safety, thereby creating opportunities for CROs specializing in this area. The integration of genomic data and biomarkers into preclinical research is expected to enhance the precision of drug development, further driving the growth of the preclinical cro market.
Rising Demand for Drug Development
The preclinical cro market in Spain is experiencing a notable surge in demand for drug development services. This trend is largely driven by the increasing number of pharmaceutical companies seeking to expedite their research and development processes. In 2025, the Spanish pharmaceutical sector is projected to invest approximately €1.5 billion in R&D, which is likely to enhance the need for preclinical services. As companies aim to bring innovative therapies to market more rapidly, the reliance on preclinical contract research organizations (CROs) is expected to grow. This rising demand not only reflects the competitive landscape of the pharmaceutical industry but also indicates a shift towards more efficient and cost-effective drug development strategies, thereby propelling the preclinical cro market forward.
Collaboration with Academic Institutions
Collaboration between preclinical CROs and academic institutions is becoming increasingly prevalent in Spain, significantly impacting the preclinical cro market. These partnerships facilitate the exchange of knowledge and resources, enabling more innovative research outcomes. In 2025, it is projected that around 40% of preclinical studies will involve collaborations with universities and research centers. Such collaborations not only enhance the quality of preclinical research but also provide access to cutting-edge technologies and methodologies. As academic institutions seek to translate their research into viable therapeutic options, the demand for preclinical services is likely to grow, thereby driving the expansion of the preclinical cro market.